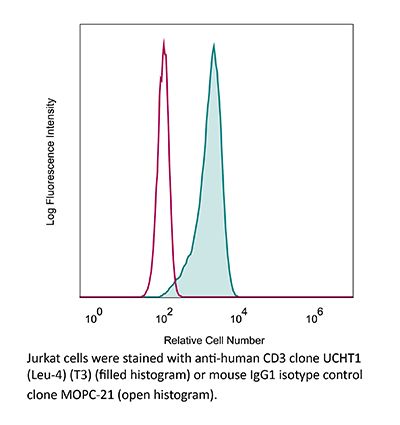
InVivoMAb anti-human CD3
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Human CD3ε |
| Reported Applications |
in vivo T cell depletion in humanized mice ex vivo T cell inhibition for xenographs Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687713 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo T cell depletion in humanized mice
ex vivo T cell inhibtion for xenografts
in vivo T cell depletion in humanized mice
ex vivo T cell inhibtion for xenografts
Wunderlich, M., et al (2014). "OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues" Blood 123(24): e134-144.
PubMed
Immunodeficient mice are now readily engrafted with human hematopoietic cells. However, these mice are susceptible to graft-versus-host disease (GVHD) induced by the engraftment and rapid expansion of coinjected human T cells. Therefore, highly purified sample populations must be used, adding significant time, expense, and effort. Here, we have explored in vivo and in vitro methods utilizing anti-T-cell antibodies to circumvent this problem. Intraperitoneal injection of the antibody within 48 hours prevented GVHD. Alternatively, short-term in vitro incubation of cells with antibody immediately before transplant was equally effective. Although in vitro antithymocyte globulin treatment resulted in a dramatic loss of SCID-repopulating cells (SRCs), treatment with OKT3 or UCHT1 abrogated GVHD risk and preserved engraftment potential. Leukemia samples that presented with substantial human T-cell contamination were effectively rescued from GVHD. In addition, OKT3 treatment of unfractionated cord blood resulted in robust engraftment of primary and secondary mice that was indistinguishable from grafts obtained using purified CD34(+) cells. Limiting dilution analysis of unfractionated blood demonstrated a SRC frequency of 1 in 300 to 500 CD34(+) cells, similar to that of purified hematopoietic stem and progenitor cells. This protocol streamlines xenograft studies while significantly reducing the cost and time of the procedure.
in vivo T cell depletion
Woo, J. H., et al (2010). "Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials" Methods Mol Biol 651: 157-175.
PubMed
Anti-CD3 recombinant diphtheria immunotoxin, A-dmDT(390)-bisFv(UCHT1), consists of the catalytic and translocation domains of diphtheria toxin fused to two single chain Fv fragments of an anti-CD3epsilon monoclonal antibody (UCHT1). A-dmDT(390)-bisFv(UCHT1) is capable of killing CD3(+) T-lymphoma cells and normal T cells specifically in the femtomolar concentration range. To study pharmacology of A-dmDT(390)-bisFv(UCHT1) in patients with CD3(+) T-cell lymphoma in a phase I clinical trial, (1) highly sensitive bioassay using Jurkat cells for measuring drug levels, (2) ELISA for measuring anti-DT antibody titer, and (3) 5-color FACS analysis method for measuring changes of subtype T-cell population were developed. In addition to evaluating drug efficacy and pharmacokinetics in patients, it is important to correlate pre-existing anti-DT antibody levels with maximum drug concentration in serum and extent of T-cell depletion because pre-existing anti-DT antibodies due to DPT (Diphtheria, Pertussis, and Tetanus) immunization can neutralize diphtheria immunotoxin. We observed that at the lowest treatment dose (2.5 microg/kg: twice daily for 4 days) A-dmDT(390)-bisFv(UCHT1) depletes greater than 99.0% of normal T cells in all six patients for a short period of time (2-3 days) and that there is no association of C (max) and extent of T-cell depletion with the pre-existing anti-DT antibody titer.
Flow Cytometry
Rossi, N. E., et al (2008). "Differential antibody binding to the surface alphabetaTCR.CD3 complex of CD4+ and CD8+ T lymphocytes is conserved in mammals and associated with differential glycosylation" Int Immunol 20(10): 1247-1258.
PubMed
We have previously shown that the surface alphabeta T cell antigen receptor (TCR).CD3 complex borne by human CD4(+) and CD8(+) T lymphocytes can be distinguished using mAbs. Using two unrelated sets of antibodies, we have now extended this finding to the surface alphabetaTCR.CD3 of seven additional mammalian species (six non-human primates and the mouse). We have also produced data supporting that differential glycosylation of the two main T cell subsets is involved in the observed TCR.CD3 antibody-binding differences in humans. First, we show differential lectin binding to human CD4(+) versus CD8(+) T lymphocytes, particularly with galectin 7. Second, we show that certain lectins can compete differentially with CD3 mAb binding to human primary CD4(+) and CD8(+) T lymphocytes. Third, N-glycan disruption using swainsonine was shown to increase mAb binding to the alphabetaTCR.CD3. We conclude that the differential antibody binding to the surface alphabetaTCR.CD3 complex of primary CD4(+) and CD8(+) T lymphocytes is phylogenetically conserved and associated with differential glycosylation. The differences may be exploited for therapeutic purposes, such as T cell lineage-specific immunosuppression of graft rejection. Also, the impact of glycosylation on CD3 antibody binding requires a cautious interpretation of CD3 expression levels and T cell numbers in clinical diagnosis.
Arnett, K. L., et al (2004). "Crystal structure of a human CD3-epsilon/delta dimer in complex with a UCHT1 single-chain antibody fragment" Proc Natl Acad Sci U S A 101(46): 16268-16273.
PubMed
The alpha/beta T cell receptor complex transmits signals from MHC/peptide antigens through a set of constitutively associated signaling molecules, including CD3-epsilon/gamma and CD3-epsilon/delta. We report the crystal structure at 1.9-A resolution of a complex between a human CD3-epsilon/delta ectodomain heterodimer and a single-chain fragment of the UCHT1 antibody. CD3-epsilon/delta and CD3-epsilon/gamma share a conserved interface between the Ig-fold ectodomains, with parallel packing of the two G strands. CD3-delta has a more electronegative surface and a more compact Ig fold than CD3-gamma; thus, the two CD3 heterodimers have distinctly different molecular surfaces. The UCHT1 antibody binds near an acidic region of CD3-epsilon opposite the dimer interface, occluding this region from direct interaction with the TCR. This immunodominant epitope may be a uniquely accessible surface in the TCR/CD3 complex, because there is overlap between the binding site of the UCHT1 and OKT3 antibodies. Determination of the CD3-epsilon/delta structure completes the set of TCR/CD3 globular ectodomains and contributes information about exposed CD3 surfaces.
in vivo T cell depletion in humanized mice
ex vivo T cell inhibtion for xenografts
in vivo T cell depletion in humanized mice
ex vivo T cell inhibtion for xenografts
Wunderlich, M., et al (2014). "OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues" Blood 123(24): e134-144.
PubMed
Immunodeficient mice are now readily engrafted with human hematopoietic cells. However, these mice are susceptible to graft-versus-host disease (GVHD) induced by the engraftment and rapid expansion of coinjected human T cells. Therefore, highly purified sample populations must be used, adding significant time, expense, and effort. Here, we have explored in vivo and in vitro methods utilizing anti-T-cell antibodies to circumvent this problem. Intraperitoneal injection of the antibody within 48 hours prevented GVHD. Alternatively, short-term in vitro incubation of cells with antibody immediately before transplant was equally effective. Although in vitro antithymocyte globulin treatment resulted in a dramatic loss of SCID-repopulating cells (SRCs), treatment with OKT3 or UCHT1 abrogated GVHD risk and preserved engraftment potential. Leukemia samples that presented with substantial human T-cell contamination were effectively rescued from GVHD. In addition, OKT3 treatment of unfractionated cord blood resulted in robust engraftment of primary and secondary mice that was indistinguishable from grafts obtained using purified CD34(+) cells. Limiting dilution analysis of unfractionated blood demonstrated a SRC frequency of 1 in 300 to 500 CD34(+) cells, similar to that of purified hematopoietic stem and progenitor cells. This protocol streamlines xenograft studies while significantly reducing the cost and time of the procedure.
in vivo T cell depletion
Woo, J. H., et al (2010). "Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials" Methods Mol Biol 651: 157-175.
PubMed
Anti-CD3 recombinant diphtheria immunotoxin, A-dmDT(390)-bisFv(UCHT1), consists of the catalytic and translocation domains of diphtheria toxin fused to two single chain Fv fragments of an anti-CD3epsilon monoclonal antibody (UCHT1). A-dmDT(390)-bisFv(UCHT1) is capable of killing CD3(+) T-lymphoma cells and normal T cells specifically in the femtomolar concentration range. To study pharmacology of A-dmDT(390)-bisFv(UCHT1) in patients with CD3(+) T-cell lymphoma in a phase I clinical trial, (1) highly sensitive bioassay using Jurkat cells for measuring drug levels, (2) ELISA for measuring anti-DT antibody titer, and (3) 5-color FACS analysis method for measuring changes of subtype T-cell population were developed. In addition to evaluating drug efficacy and pharmacokinetics in patients, it is important to correlate pre-existing anti-DT antibody levels with maximum drug concentration in serum and extent of T-cell depletion because pre-existing anti-DT antibodies due to DPT (Diphtheria, Pertussis, and Tetanus) immunization can neutralize diphtheria immunotoxin. We observed that at the lowest treatment dose (2.5 microg/kg: twice daily for 4 days) A-dmDT(390)-bisFv(UCHT1) depletes greater than 99.0% of normal T cells in all six patients for a short period of time (2-3 days) and that there is no association of C (max) and extent of T-cell depletion with the pre-existing anti-DT antibody titer.
Flow Cytometry
Rossi, N. E., et al (2008). "Differential antibody binding to the surface alphabetaTCR.CD3 complex of CD4+ and CD8+ T lymphocytes is conserved in mammals and associated with differential glycosylation" Int Immunol 20(10): 1247-1258.
PubMed
We have previously shown that the surface alphabeta T cell antigen receptor (TCR).CD3 complex borne by human CD4(+) and CD8(+) T lymphocytes can be distinguished using mAbs. Using two unrelated sets of antibodies, we have now extended this finding to the surface alphabetaTCR.CD3 of seven additional mammalian species (six non-human primates and the mouse). We have also produced data supporting that differential glycosylation of the two main T cell subsets is involved in the observed TCR.CD3 antibody-binding differences in humans. First, we show differential lectin binding to human CD4(+) versus CD8(+) T lymphocytes, particularly with galectin 7. Second, we show that certain lectins can compete differentially with CD3 mAb binding to human primary CD4(+) and CD8(+) T lymphocytes. Third, N-glycan disruption using swainsonine was shown to increase mAb binding to the alphabetaTCR.CD3. We conclude that the differential antibody binding to the surface alphabetaTCR.CD3 complex of primary CD4(+) and CD8(+) T lymphocytes is phylogenetically conserved and associated with differential glycosylation. The differences may be exploited for therapeutic purposes, such as T cell lineage-specific immunosuppression of graft rejection. Also, the impact of glycosylation on CD3 antibody binding requires a cautious interpretation of CD3 expression levels and T cell numbers in clinical diagnosis.
Arnett, K. L., et al (2004). "Crystal structure of a human CD3-epsilon/delta dimer in complex with a UCHT1 single-chain antibody fragment" Proc Natl Acad Sci U S A 101(46): 16268-16273.
PubMed
The alpha/beta T cell receptor complex transmits signals from MHC/peptide antigens through a set of constitutively associated signaling molecules, including CD3-epsilon/gamma and CD3-epsilon/delta. We report the crystal structure at 1.9-A resolution of a complex between a human CD3-epsilon/delta ectodomain heterodimer and a single-chain fragment of the UCHT1 antibody. CD3-epsilon/delta and CD3-epsilon/gamma share a conserved interface between the Ig-fold ectodomains, with parallel packing of the two G strands. CD3-delta has a more electronegative surface and a more compact Ig fold than CD3-gamma; thus, the two CD3 heterodimers have distinctly different molecular surfaces. The UCHT1 antibody binds near an acidic region of CD3-epsilon opposite the dimer interface, occluding this region from direct interaction with the TCR. This immunodominant epitope may be a uniquely accessible surface in the TCR/CD3 complex, because there is overlap between the binding site of the UCHT1 and OKT3 antibodies. Determination of the CD3-epsilon/delta structure completes the set of TCR/CD3 globular ectodomains and contributes information about exposed CD3 surfaces.
in vivo T cell depletion in humanized mice
ex vivo T cell inhibtion for xenografts
Wunderlich, M., et al (2014). "OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues" Blood 123(24): e134-144.
PubMed
Immunodeficient mice are now readily engrafted with human hematopoietic cells. However, these mice are susceptible to graft-versus-host disease (GVHD) induced by the engraftment and rapid expansion of coinjected human T cells. Therefore, highly purified sample populations must be used, adding significant time, expense, and effort. Here, we have explored in vivo and in vitro methods utilizing anti-T-cell antibodies to circumvent this problem. Intraperitoneal injection of the antibody within 48 hours prevented GVHD. Alternatively, short-term in vitro incubation of cells with antibody immediately before transplant was equally effective. Although in vitro antithymocyte globulin treatment resulted in a dramatic loss of SCID-repopulating cells (SRCs), treatment with OKT3 or UCHT1 abrogated GVHD risk and preserved engraftment potential. Leukemia samples that presented with substantial human T-cell contamination were effectively rescued from GVHD. In addition, OKT3 treatment of unfractionated cord blood resulted in robust engraftment of primary and secondary mice that was indistinguishable from grafts obtained using purified CD34(+) cells. Limiting dilution analysis of unfractionated blood demonstrated a SRC frequency of 1 in 300 to 500 CD34(+) cells, similar to that of purified hematopoietic stem and progenitor cells. This protocol streamlines xenograft studies while significantly reducing the cost and time of the procedure.
in vivo T cell depletion
Woo, J. H., et al (2010). "Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials" Methods Mol Biol 651: 157-175.
PubMed
Anti-CD3 recombinant diphtheria immunotoxin, A-dmDT(390)-bisFv(UCHT1), consists of the catalytic and translocation domains of diphtheria toxin fused to two single chain Fv fragments of an anti-CD3epsilon monoclonal antibody (UCHT1). A-dmDT(390)-bisFv(UCHT1) is capable of killing CD3(+) T-lymphoma cells and normal T cells specifically in the femtomolar concentration range. To study pharmacology of A-dmDT(390)-bisFv(UCHT1) in patients with CD3(+) T-cell lymphoma in a phase I clinical trial, (1) highly sensitive bioassay using Jurkat cells for measuring drug levels, (2) ELISA for measuring anti-DT antibody titer, and (3) 5-color FACS analysis method for measuring changes of subtype T-cell population were developed. In addition to evaluating drug efficacy and pharmacokinetics in patients, it is important to correlate pre-existing anti-DT antibody levels with maximum drug concentration in serum and extent of T-cell depletion because pre-existing anti-DT antibodies due to DPT (Diphtheria, Pertussis, and Tetanus) immunization can neutralize diphtheria immunotoxin. We observed that at the lowest treatment dose (2.5 microg/kg: twice daily for 4 days) A-dmDT(390)-bisFv(UCHT1) depletes greater than 99.0% of normal T cells in all six patients for a short period of time (2-3 days) and that there is no association of C (max) and extent of T-cell depletion with the pre-existing anti-DT antibody titer.
Flow Cytometry
Rossi, N. E., et al (2008). "Differential antibody binding to the surface alphabetaTCR.CD3 complex of CD4+ and CD8+ T lymphocytes is conserved in mammals and associated with differential glycosylation" Int Immunol 20(10): 1247-1258.
PubMed
We have previously shown that the surface alphabeta T cell antigen receptor (TCR).CD3 complex borne by human CD4(+) and CD8(+) T lymphocytes can be distinguished using mAbs. Using two unrelated sets of antibodies, we have now extended this finding to the surface alphabetaTCR.CD3 of seven additional mammalian species (six non-human primates and the mouse). We have also produced data supporting that differential glycosylation of the two main T cell subsets is involved in the observed TCR.CD3 antibody-binding differences in humans. First, we show differential lectin binding to human CD4(+) versus CD8(+) T lymphocytes, particularly with galectin 7. Second, we show that certain lectins can compete differentially with CD3 mAb binding to human primary CD4(+) and CD8(+) T lymphocytes. Third, N-glycan disruption using swainsonine was shown to increase mAb binding to the alphabetaTCR.CD3. We conclude that the differential antibody binding to the surface alphabetaTCR.CD3 complex of primary CD4(+) and CD8(+) T lymphocytes is phylogenetically conserved and associated with differential glycosylation. The differences may be exploited for therapeutic purposes, such as T cell lineage-specific immunosuppression of graft rejection. Also, the impact of glycosylation on CD3 antibody binding requires a cautious interpretation of CD3 expression levels and T cell numbers in clinical diagnosis.
Arnett, K. L., et al (2004). "Crystal structure of a human CD3-epsilon/delta dimer in complex with a UCHT1 single-chain antibody fragment" Proc Natl Acad Sci U S A 101(46): 16268-16273.
PubMed
The alpha/beta T cell receptor complex transmits signals from MHC/peptide antigens through a set of constitutively associated signaling molecules, including CD3-epsilon/gamma and CD3-epsilon/delta. We report the crystal structure at 1.9-A resolution of a complex between a human CD3-epsilon/delta ectodomain heterodimer and a single-chain fragment of the UCHT1 antibody. CD3-epsilon/delta and CD3-epsilon/gamma share a conserved interface between the Ig-fold ectodomains, with parallel packing of the two G strands. CD3-delta has a more electronegative surface and a more compact Ig fold than CD3-gamma; thus, the two CD3 heterodimers have distinctly different molecular surfaces. The UCHT1 antibody binds near an acidic region of CD3-epsilon opposite the dimer interface, occluding this region from direct interaction with the TCR. This immunodominant epitope may be a uniquely accessible surface in the TCR/CD3 complex, because there is overlap between the binding site of the UCHT1 and OKT3 antibodies. Determination of the CD3-epsilon/delta structure completes the set of TCR/CD3 globular ectodomains and contributes information about exposed CD3 surfaces.