InVivoMAb anti-mouse IL-6
Product Details
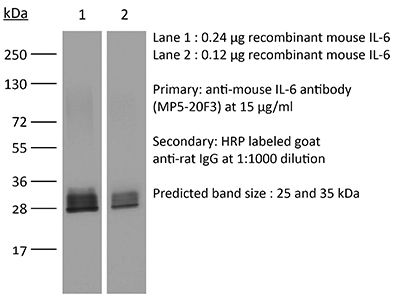
The MP5-20F3 monoclonal antibody reacts with mouse IL-6 (interleukin-6) a 21-28 kDa cytokine that is expressed by many cell types, including T lymphocytes, B lymphocytes, monocytes, fibroblasts, and endothelial cells. IL-6 signals through a cell-surface type I cytokine receptor complex consisting of the ligand-binding IL-6Rα chain (CD126), and the signal-transducing component gp130 (also called CD130). Upon receptor binding IL-6 influences antigen-specific immune responses, inflammatory responses, neuronal development, and is a major mediator of the acute phase reaction. The MP5-20F3 monoclonal antibody has been shown to neutralize the bioactivity of natural or recombinant IL-6.Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Immunogen | Recombinant mouse IL-6 |
| Reported Applications |
in vivo IL-6 neutralization in vitro IL-6 neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Sterility | 0.2 μM filtered |
| Production | Purified from tissue culture supernatant in an animal free facility |
| Purification | Protein G |
| RRID | AB_1107709 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo IL-12p40 neutralization, in vivo IL-6 neutralization
Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus PubMed
Staphylococcus aureus is a common cause of prosthetic implant infections, which can become chronic due to the ability of S. aureus to grow as a biofilm. Little is known about adaptive immune responses to these infections in vivo. We hypothesized that S. aureus elicits inflammatory Th1/Th17 responses, associated with biofilm formation, instead of protective Th2/Treg responses. We used an adapted mouse model of biofilm-mediated prosthetic implant infection to determine chronic infection rates, Treg cell frequencies, and local cytokine levels in Th1-biased C57BL/6 and Th2-biased BALB/c mice. All C57BL/6 mice developed chronic S. aureus implant infection at all time points tested. However, over 75% of BALB/c mice spontaneously cleared the infection without adjunctive therapy and demonstrated higher levels of Th2 cytokines and anti-inflammatory Treg cells. When chronic infection rates in mice deficient in the Th2 cytokine interleukin-4 (IL-4) via STAT6 mutation in a BALB/c background were assessed, the mice were unable to clear the S. aureus implant infection. Additionally, BALB/c mice depleted of Treg cells via an anti-CD25 monoclonal antibody (MAb) were also unable to clear the infection. In contrast, the C57BL/6 mice that were susceptible to infection were able to eliminate S. aureus biofilm populations on infected intramedullary pins once the Th1 and Th17 responses were diminished by MAb treatment with anti-IL-12 p40. Together, these results indicate that Th2/Treg responses are mechanisms of protection against chronic S. aureus implant infection, as opposed to Th1/Th17 responses, which may play a role in the development of chronic infection.
in vitro IL-6 neutralization
High TCR stimuli prevent induced regulatory T cell differentiation in a NF-kappaB-dependent manner PubMed
The concentration of Ag or mitogenic stimuli is known to play an important role in controlling the differentiation of naive CD4(+) T cells into different effector phenotypes. In particular, whereas TCR engagement at low Ag doses in the presence of TGF-beta and IL-2 can promote differentiation of Foxp3-expressing induced regulatory T cells (iTregs), high levels of Ag have been shown in vitro and in vivo to prevent Foxp3 upregulation. This tight control of iTreg differentiation dictated by Ag dose most likely determines the quality and duration of an immune response. However, the molecular mechanism by which this high-dose inhibition of Foxp3 induction occurs is not well understood. In this study, we demonstrate that when cells are in the presence of CD28 costimulation, TCR-dependent NF-kappaB signaling is essential for Foxp3 inhibition at high doses of TCR engagement in mouse T cells. Prevention of Foxp3 induction depends on the production of NF-kappaB-dependent cytokines by the T cells themselves. Moreover, T cells that fail to upregulate Foxp3 under iTreg-differentiating conditions and high TCR stimulation acquire the capacity to make TNF and IFN-gamma, as well as IL-17 and IL-9. Thus, NF-kappaB helps T cells control their differentiation fate in a cell-intrinsic manner and prevents peripheral iTreg development under conditions of high Ag load that may require more vigorous effector T cell responses.
in vivo IL-6 neutralization
Th17 alloimmunity prevents neonatal establishment of lymphoid chimerism in IL-4-deprived mice PubMed
Immune responses in newborn mice are known to be biased toward the helper type 2 phenotype. This may account for their propensity to develop tolerance. Herein, we evaluated the effects of IL-4 deprivation on CD4(+) T-cell activities elicited by neonatal exposure to allogeneic spleen cells. We showed that chimerism, Th2-type polarization and pathology, as well as skin allograft acceptance were inhibited in BALB/c mice immunized at birth with (A/J x BALB/c) F(1) spleen cells upon in vivo IL-4 neutralization. While IL-4 neutralization inhibited the development of Th2 cells in this model, it led to the accumulation of IL-17A, IL-17F, IL-22, IL-6 and RORgammat mRNA in the spleen or graft tissues. Moreover, IL-4 deprivation led to the differentiation of donor-specific Th17 cells with a concomitant Th1 response characterized by IFN-gamma production. The Th17-type response emerging in IL-4-deprived mice was found to mediate both intragraft neutrophil infiltration and the abrogation of B-cell chimerism. Neutralization of this Th17 response failed however to restore functional skin graft acceptance. Collectively, our observations indicate that the neonatal Th2 response opposes the development of Th17 cells, and that Th17 cells are responsible for controlling lymphoid chimerism in mice neonatally injected with semiallogeneic cells.
in vitro T cell stimulation/activation, in vivo IL-17A neutralization, in vivo IL-6 neutralization
SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
Flow Cytometry, in vivo IFNγ neutralization, in vivo IL-6 neutralization, in vivo TNFα neutralization
CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection PubMed
Synthetic glucocorticoids (GCs) are commonly used in the treatment of inflammatory diseases, but the role of endogenous GCs in the regulation of host-protective immune responses is poorly understood. Here we show that GCs are induced during acute Toxoplasma gondii infection and directly control the T cell response to the parasite. When infected with toxoplasma, mice that selectively lack GC receptor (GR) expression in T cells (GR(lck-Cre)) rapidly succumb to infection despite displaying parasite burdens indistinguishable from control animals and unaltered levels of the innate cytokines IL-12 and IL-27. Mortality in the GR(lck-Cre) mice was associated with immunopathology and hyperactive Th1 cell function as revealed by enhanced IFN-gamma and TNF production in vivo. Unexpectedly, these CD4(+) T lymphocytes also overexpressed IL-10. Importantly, CD4(+) T cell depletion in wild-type or GR(lck-Cre) mice led to ablation of the GC response to infection. Moreover, in toxoplasma-infected RAG(-/-) animals, adoptive transfer of CD4(+) T lymphocytes was required for GC induction. These findings establish a novel IL-10-independent immunomodulatory circuit in which CD4(+) T cells trigger a GC response that in turn dampens their own effector function. In the case of T. gondii infection, this self-regulatory pathway is critical for preventing collateral tissue damage and promoting host survival.
in vivo blocking of IL-6/IL-6R signaling, in vivo IL-6 neutralization
Role of IL-6 in Mycobacterium avium–associated immune reconstitution inflammatory syndrome PubMed
Immune reconstitution inflammatory syndrome (IRIS) is a major adverse event of antiretroviral therapy in HIV infection, and paradoxically occurs as HIV viremia is suppressed and CD4 T cell numbers recover. IRIS reflects pathogenic immune responses against opportunistic infections acquired during the period of immunodeficiency, but little is understood about the mechanisms of inflammatory pathology. In this study, we show that IL-6 and C-reactive protein levels transiently rise at the time of the IRIS event in HIV-infected patients, unmasking Mycobacterium avium complex infection after starting antiretroviral therapy. To directly test the role of IL-6 in IRIS pathology, we used a model of experimentally inducible IRIS in which M. avium-infected T cell-deficient mice undergo a fatal inflammatory disease after reconstitution with CD4 T cells. We find that IL-6 neutralization reduces C-reactive protein levels, alleviates wasting disease, and extends host survival during experimental IRIS. Moreover, we show that combined blockade of IL-6 and IFN-gamma further reduces IRIS pathology, even after the onset of wasting disease. The combination of these clinical and experimental-model data show that the IL-6 pathway is not only a biomarker of mycobacterial IRIS but also a major mediator of pathology distinct from IFN-gamma and may be a useful target for therapeutic intervention.
Flow Cytometry, in vivo depletion of Gr-1+ myeloid cells, in vivo IL-17A neutralization, in vivo IL-1β neutralization, in vivo IL-6 neutralization, in vivo TNFα neutralization
Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vitro IL-6 neutralization
Mesenchymal stem cells exert anti-proliferative effect on lipopolysaccharide-stimulated BV2 microglia by reducing tumour necrosis factor-alpha levels PubMed
BACKGROUND: Progression of neurodegenerative diseases occurs when microglia, upon persistent activation, perpetuate a cycle of damage in the central nervous system. Use of mesenchymal stem cells (MSC) has been suggested as an approach to manage microglia activation based on their immunomodulatory functions. In the present study, we describe the mechanism through which bone marrow-derived MSC modulate the proliferative responses of lipopolysaccharide-stimulated BV2 microglia. METHODS: BV2 microglia were cultured with MSC and stimulated with 1 mug/ml lipopolysaccharide. Using an inducible nitric oxide synthase inhibitor, tritiated thymidine (3H-TdR) incorporation assay was performed to determine the role of nitric oxide in the anti-proliferative effect of MSC. We also studied apoptosis and the cell cycle of both cell types using flow cytometry and explored their cytokine profile using protein and cytometric arrays. Moreover, the role of IL-6 and TNF-alpha in immunomodulation was deduced using specific blocking antibodies and recombinant proteins. RESULTS: MSC reduces microglia proliferation upon lipopolysaccharide stimulation by 21 to 28% and modulates the levels of nitric oxide, IL-6 and TNF-alpha. The role of nitric oxide in conferring the anti-proliferative effect of MSC was ruled out. Furthermore, we found that MSC exert their anti-proliferative effect by restoring the percentage of BV2 cells at S and G2/M phase to levels similar to unstimulated cells. MSC undergo a G0/G1 arrest while exerting this effect. We have also identified that MSC-mediated modulation of microglia is independent of IL-6, whilst reduction of TNF-alpha in co-culture is critical for inhibition of microglia proliferation. CONCLUSIONS: Our study demonstrates that MSC inhibit microglia proliferation independent of nitric oxide and IL-6, although reduction of TNF-alpha is critical for this effect. The inhibition of proliferation is through cell cycle modulation. These findings shed light on the mechanisms of microglial immunomodulation by MSC.
in vivo IFNγ neutralization, in vivo IL-6 neutralization, in vivo LFA-1 neutralization
Donor CD4 T cells trigger costimulation blockade-resistant donor bone marrow rejection through bystander activation requiring IL-6 PubMed
Bone marrow (BM) transplantation under costimulation blockade induces chimerism and tolerance. Cotransplantation of donor T cells (contained in substantial numbers in mobilized peripheral blood stem cells and donor lymphocyte infusions) together with donor BM paradoxically triggers rejection of donor BM through undefined mechanisms. Here, nonmyeloablatively irradiated C57BL/6 recipients simultaneously received donor BM (BALB/c) and donor T cells under costimulation blockade (anti-CD154 and CTLA4Ig). Donor CD4, but not CD8 cells, triggered natural killer-independent donor BM rejection which was associated with increased production of IL-6, interferon gamma (IFN-gamma) and IL-17A. BM rejection was prevented through neutralization of IL-6, but not of IFN-gamma or IL-17A. IL-6 counteracted the antiproliferative effect of anti-CD154 in vitro. Rapamycin and anti-lymphocyte function-associated antigen 1 negated this effect of IL-6 in vitro and prevented BM rejection in vivo. Simultaneous cotransplantation of (BALB/cxB6)F1, recipient or irradiated donor CD4 cells, or late transfer of donor CD4 cells did not lead to BM rejection, whereas cotransplantation of third party CD4 cells did. Transferred donor CD4 cells became activated, rapidly underwent apoptosis and triggered activation and proliferation of recipient T cells. Collectively, these results provide evidence that donor T cells recognizing the recipient as allogeneic lead to the release of IL-6, which abolishes the effect of anti-CD154, triggering donor BM rejection through bystander activation.
in vivo blocking of IL-6/IL-6R signaling, in vivo IL-10 neutralization, in vivo IL-6 neutralization
IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age PubMed
Decline in immune function and inflammation concomitantly develop with ageing. Here we focus on the impact of this inflammatory environment on T cells, and demonstrate that in contrast to successful tumour elimination in young mice, replenishment of tumour-specific CD4(+) T cells fails to induce tumour regression in aged hosts. The impaired antitumour effect of CD4(+) T cells with their defective Th1 differentiation in an aged environment is restored by interleukin (IL)-6 blockade or IL-6 deficiency. IL-6 blockade also restores the impaired ability of CD4(+) T cells to promote CD8(+) T-cell-dependent tumour elimination in aged mice, which requires IFN-gamma. Furthermore, IL-6-stimulated production of IL-4/IL-21 through c-Maf induction is responsible for impaired Th1 differentiation. IL-6 also contributes to IL-10 production from CD4(+) T cells in aged mice, causing attenuated responses of CD8(+) T cells. These findings suggest that IL-6 serves as an extrinsic factor counteracting CD4(+) T-cell-mediated immunity against tumour in old age.
in vivo IL-6 neutralization
Innate lymphotoxin receptor mediated signaling promotes HSV-1 associated neuroinflammation and viral replication PubMed
Host anti-viral innate immunity plays important roles in the defense against HSV-1 infection. In this study, we find an unexpected role for innate LT/LIGHT signaling in promoting HSV-1 replication and virus induced inflammation in immunocompromised mice. Using a model of footpad HSV-1 infection in Rag1(-/-) mice, we observed that blocking LT/LIGHT signaling with LTbetaR-Ig could significantly delay disease progression and extend the survival of infected mice. LTbetaR-Ig treatment reduced late proinflammatory cytokine release in the serum and nervous tissue, and inhibited chemokine expression and inflammatory cells infiltration in the dorsal root ganglia (DRG). Intriguingly, LTbetaR-Ig treatment restricted HSV-1 replication in the DRG but not the footpad. These findings demonstrate a critical role for LT/LIGHT signaling in modulating innate inflammation and promoting HSV-1 replication in the nervous system, and suggest a new target for treatment of virus-induced adverse immune response and control of severe HSV-1 infection.
Flow Cytometry, in vivo IL-6 neutralization
IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment PubMed
The aggressiveness of invasive ductal carcinoma (IDC) of the breast is associated with increased IL17 levels. Studying the role of IL17 in invasive breast tumor pathogenesis, we found that metastatic primary tumor-infiltrating T lymphocytes produced elevated levels of IL17, whereas IL17 neutralization inhibited tumor growth and prevented the migration of neutrophils and tumor cells to secondary disease sites. Tumorigenic neutrophils promote disease progression, producing CXCL1, MMP9, VEGF, and TNFalpha, and their depletion suppressed tumor growth. IL17A also induced IL6 and CCL20 production in metastatic tumor cells, favoring the recruitment and differentiation of Th17. In addition, IL17A changed the gene-expression profile and the behavior of nonmetastatic tumor cells, causing tumor growth in vivo, confirming the protumor role of IL17. Furthermore, high IL17 expression was associated with lower disease-free survival and worse prognosis in IDC patients. Thus, IL17 blockade represents an attractive approach for the control of invasive breast tumors. Cancer Res; 75(18); 3788-99. (c)2015 AACR.