InVivoMAb anti-mouse VISTA
Product Details
The 13F3 monoclonal antibody reacts with mouse V-domain Ig suppressor of T cell activation (VISTA) also known as PD-1H and B7-H5. VISTA is a 309 aa type I transmembrane glycoprotein and a member of the Ig superfamily. VISTA is expressed on naïve and activated T cells, NK cells, macrophages, dendritic cells, and neutrophils. VISTA functions as a negative immune-checkpoint protein that suppresses T cell cytokine production and proliferation. VISTA is overexpressed by tumor-infiltrating lymphocytes, such as myeloid cells and regulatory T cells. Blockade of VISTA with the 13F3 antibody results in delayed tumor growth in mouse models of melanoma.Specifications
| Isotype | Armenian hamster IgG |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
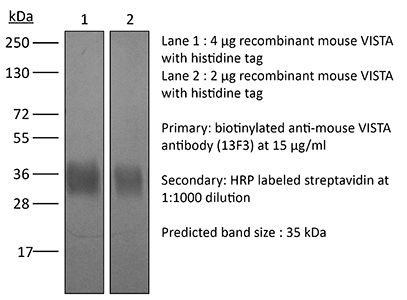
| Immunogen | EL4 cells overexpressing mouse VISTA-RFP and then boosted with VISTA-Ig fusion protein |
| Reported Applications |
in vivo blocking of VISTA signaling in vitro blocking of VISTA signaling |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Sterility | 0.2 μM filtered |
| Production | Purified from tissue culture supernatant in an animal free facility |
| Purification | Protein A |
| RRID | AB_2736990 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vitro VISTA neutralization, in vivo VISTA neutralization
VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses PubMed
The immunoglobulin (Ig) superfamily consists of many critical immune regulators, including the B7 family ligands and receptors. In this study, we identify a novel and structurally distinct Ig superfamily inhibitory ligand, whose extracellular domain bears homology to the B7 family ligand PD-L1. This molecule is designated V-domain Ig suppressor of T cell activation (VISTA). VISTA is primarily expressed on hematopoietic cells, and VISTA expression is highly regulated on myeloid antigen-presenting cells (APCs) and T cells. A soluble VISTA-Ig fusion protein or VISTA expression on APCs inhibits T cell proliferation and cytokine production in vitro. A VISTA-specific monoclonal antibody interferes with VISTA-induced suppression of T cell responses by VISTA-expressing APCs in vitro. Furthermore, anti-VISTA treatment exacerbates the development of the T cell-mediated autoimmune disease experimental autoimmune encephalomyelitis in mice. Finally, VISTA overexpression on tumor cells interferes with protective antitumor immunity in vivo in mice. These findings show that VISTA, a novel immunoregulatory molecule, has functional activities that are nonredundant with other Ig superfamily members and may play a role in the development of autoimmunity and immune surveillance in cancer.
in vivo VISTA neutralization
VISTA Regulates the Development of Protective Antitumor Immunity PubMed
V-domain Ig suppressor of T-cell activation (VISTA) is a novel negative checkpoint ligand that is homologous to PD-L1 and suppresses T-cell activation. This study demonstrates the multiple mechanisms whereby VISTA relieves negative regulation by hematopoietic cells and enhances protective antitumor immunity. VISTA is highly expressed on myeloid cells and Foxp3(+)CD4(+) regulatory cells, but not on tumor cells within the tumor microenvironment (TME). VISTA monoclonal antibody (mAb) treatment increased the number of tumor-specific T cells in the periphery and enhanced the infiltration, proliferation, and effector function of tumor-reactive T cells within the TME. VISTA blockade altered the suppressive feature of the TME by decreasing the presence of monocytic myeloid-derived suppressor cells and increasing the presence of activated dendritic cells within the tumor microenvironment. In addition, VISTA blockade impaired the suppressive function and reduced the emergence of tumor-specific Foxp3(+)CD4(+) regulatory T cells. Consequently, VISTA mAb administration as a monotherapy significantly suppressed the growth of both transplantable and inducible melanoma. Initial studies explored a combinatorial regimen using VISTA blockade and a peptide-based cancer vaccine with TLR agonists as adjuvants. VISTA blockade synergized with the vaccine to effectively impair the growth of established tumors. Our study therefore establishes a foundation for designing VISTA-targeted approaches either as a monotherapy or in combination with additional immune-targeted strategies for cancer immunotherapy.
Flow Cytometry
VISTA Deficiency Accelerates the Development of Fatal Murine Lupus Nephritis PubMed
OBJECTIVE: The targeting of negative checkpoint regulators as a means of augmenting antitumor immune responses is now an increasingly used and remarkably effective approach to the treatment of several human malignancies. The negative checkpoint regulator VISTA (V-domain Ig-containing suppressor of T cell activation; also known as programmed death 1 homolog or as death domain 1alpha) suppresses T cell responses and regulates myeloid activities. We proposed that exploitation of the VISTA pathway is a novel strategy for the treatment of human autoimmune disease, and therefore we undertook this study to determine the impact of VISTA genetic deficiency on lupus development in a lupus-prone mouse strain. METHODS: To evaluate whether genetic deficiency of VISTA affects the development of lupus, we interbred VISTA-deficient mice with Sle1.Sle3 mice, a well-characterized model of systemic lupus erythematosus (SLE). RESULTS: We demonstrated that the development of proteinuria and glomerulonephritis in these mice, designated Sle1.Sle3 VISTA(-/-) mice, was greatly accelerated and more severe compared to that in Sle1.Sle3 and C57BL/6 VISTA(-/-) mice. Analysis of cells from Sle1.Sle3 VISTA(-/-) mice showed enhanced activation of splenic CD4+ T cells and myeloid cell populations. No increase in titers of autoantibodies was seen in Sle1.Sle3 VISTA(-/-) mice. Most striking was a significant increase in proinflammatory cytokines, chemokines, and interferon (IFN)-regulated genes associated with SLE, such as IFNalpha, IFNgamma, tumor necrosis factor, interleukin-10, and CXCL10, in Sle1.Sle3 VISTA(-/-) mice. CONCLUSION: This study demonstrates for the first time that loss of VISTA in murine SLE exacerbates disease due to enhanced myeloid and T cell activation and cytokine production, including a robust IFNalpha signature, and supports a strategy of enhancement of the immunosuppressive activity of VISTA for the treatment of human lupus.
Flow Cytometry
CXCL17 Chemokine-Dependent Mobilization of CXCR8(+)CD8(+) Effector Memory and Tissue-Resident Memory T Cells in the Vaginal Mucosa Is Associated with Protection against Genital Herpes PubMed
Circulating conventional memory CD8(+) T cells (i.e., the CD8(+) effector memory T [TEM] cell and CD8(+) central memory T [TCM] cell subsets) and the noncirculating CD8(+) tissue-resident memory T (TRM) cell subset play a critical role in mucosal immunity. Mucosal chemokines, including the recently discovered CXCL17, are also important in mucosal immunity because they are homeostatically expressed in mucosal tissues. However, whether the CXCL17 chemokine contributes to the mobilization of memory CD8(+) T cell subsets to access infected mucosal tissues remains to be elucidated. In this study, we report that after intravaginal HSV type 1 infection of B6 mice, we detected high expression levels of CXCL17 and increased numbers of CD44(high)CD62L(low)CD8(+) TEM and CD103(high)CD8(+) TRM cells expressing CXCR8, the cognate receptor of CXCL17, in the vaginal mucosa (VM) of mice with reduced genital herpes infection and disease. In contrast to wild-type B6 mice, the CXCL17(-/-) mice developed 1) fewer CXCR8(+)CD8(+) TEM and TRM cells associated with more virus replication in the VM and more latency established in dorsal root ganglia, and 2) reduced numbers and frequencies of functional CD8(+) T cells in the VM. These findings suggest that the CXCL17/CXCR8 chemokine pathway plays a crucial role in mucosal vaginal immunity by promoting the mobilization of functional protective CD8(+) TEM and CD8(+) TRM cells, within this site of acute and recurrent herpes infection.
in vivo VISTA neutralization
Blocking the VISTA pathway enhances disease progression in (NZB x NZW) F1 female mice PubMed
V-domain Ig suppressor of T-cell activation (VISTA) is a critical negative checkpoint molecule involved in regulating the immune response. Targeting the pathway with an antagonist anti-VISTA antibody designated 13F3 has been shown to enhance disease severity in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. To determine if VISTA plays a role in murine lupus, New Zealand Black x New Zealand White (BWF1) mice were treated with 13F3 or control hamster Ig and disease monitored. Onset of proteinuria was earlier and renal damage more profound in mice treated with 13F3. Cell subset analysis showed an increase of activated splenic T cells and inflammatory splenic myeloid cells, but no effect on B cells, in mice receiving 13F3. Examination of the kidney showed an increase in inflammatory myeloid cell infiltration with 13F3 treatment. This study along with previous EAE data, suggests that interventions that enhance VISTA regulatory activity may be effective for the treatment of autoimmune disease.
in vivo VISTA neutralization
FOXD3 Regulates VISTA Expression in Melanoma PubMed
Immune checkpoint inhibitors have improved patient survival in melanoma, but the innate resistance of many patients necessitates the investigation of alternative immune targets. Many immune checkpoint proteins lack proper characterization, including V-domain Ig suppressor of T cell activation (VISTA). VISTA expression on immune cells can suppress T cell activity; however, few studies have investigated its expression and regulation in cancer cells. In this study, we observe that VISTA is expressed in melanoma patient samples and cell lines. Tumor cell-specific expression of VISTA promotes tumor onset in vivo, associated with increased intratumoral T regulatory cells, and enhanced PDL-1 expression on tumor-infiltrating macrophages. VISTA transcript levels are regulated by the stemness factor Forkhead box D3 (FOXD3). BRAF inhibition upregulates FOXD3 and reduces VISTA expression. Overall, this study demonstrates melanoma cell expression of VISTA and its regulation by FOXD3, contributing to the rationale for therapeutic strategies that combine targeted inhibitors with immune checkpoint blockade.