InVivoPlus anti-mouse IL-4
Product Description
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoPlus rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
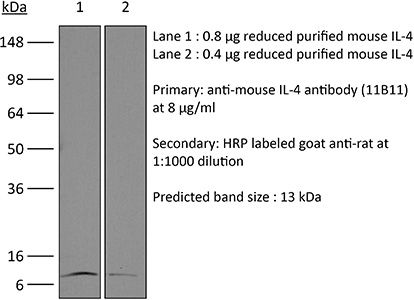
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Partially purified native mouse IL-4 |
| Reported Applications |
in vivo IL-4 neutralization in vitro IL-4 neutralization in vivo IL-4 receptor stimulation (as a complex with IL-4) Flow cytometry Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin* |
≤0.5EU/mg (≤0.0005EU/μg) Determined by LAL assay |
| Aggregation* | <5%, Determined by SEC |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107707 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Tests* |
Ectromelia/Mousepox Virus: Negative Hantavirus: Negative K Virus: Negative Lactate Dehydrogenase-Elevating Virus: Negative Lymphocytic Choriomeningitis virus: Negative Mouse Adenovirus: Negative Mouse Cytomegalovirus: Negative Mouse Hepatitis Virus: Negative Mouse Minute Virus: Negative Mouse Norovirus: Negative Mouse Parvovirus: Negative Mouse Rotavirus: Negative Mycoplasma Pulmonis: Negative Pneumonia Virus of Mice: Negative Polyoma Virus: Negative Reovirus Screen: Negative Sendai Virus: Negative Theiler’s Murine Encephalomyelitis: Negative |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Flow Cytometry
in vitro IL-4 neutralization
Burton, B. R., et al (2014). "Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy" Nat Commun 5: 4741.
PubMed
Antigen-specific immunotherapy combats autoimmunity or allergy by reinstating immunological tolerance to target antigens without compromising immune function. Optimization of dosing strategy is critical for effective modulation of pathogenic CD4(+) T-cell activity. Here we report that dose escalation is imperative for safe, subcutaneous delivery of the high self-antigen doses required for effective tolerance induction and elicits anergic, interleukin (IL)-10-secreting regulatory CD4(+) T cells. Analysis of the CD4(+) T-cell transcriptome, at consecutive stages of escalating dose immunotherapy, reveals progressive suppression of transcripts positively regulating inflammatory effector function and repression of cell cycle pathways. We identify transcription factors, c-Maf and NFIL3, and negative co-stimulatory molecules, LAG-3, TIGIT, PD-1 and TIM-3, which characterize this regulatory CD4(+) T-cell population and whose expression correlates with the immunoregulatory cytokine IL-10. These results provide a rationale for dose escalation in T-cell-directed immunotherapy and reveal novel immunological and transcriptional signatures as surrogate markers of successful immunotherapy.
in vitro IL-12 p35 neutralization
in vivo IL-12p40 neutralization
in vitro T cell stimulation/activation
Flow Cytometry
in vitro IL-4 neutralization
in vitro T cell stimulation/activation
Tang, W., et al (2014). "The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells" Immunity 41(4): 555-566.
PubMed
Bcl-3 is an atypical member of the IkappaB family that modulates transcription in the nucleus via association with p50 (NF-kappaB1) or p52 (NF-kappaB2) homodimers. Despite evidence attesting to the overall physiologic importance of Bcl-3, little is known about its cell-specific functions or mechanisms. Here we demonstrate a T-cell-intrinsic function of Bcl-3 in autoimmunity. Bcl-3-deficient T cells failed to induce disease in T cell transfer-induced colitis and experimental autoimmune encephalomyelitis. The protection against disease correlated with a decrease in Th1 cells that produced the cytokines IFN-gamma and GM-CSF and an increase in Th17 cells. Although differentiation into Th1 cells was not impaired in the absence of Bcl-3, differentiated Th1 cells converted to less-pathogenic Th17-like cells, in part via mechanisms involving expression of the RORgammat transcription factor. Thus, Bcl-3 constrained Th1 cell plasticity and promoted pathogenicity by blocking conversion to Th17-like cells, revealing a unique type of regulation that shapes adaptive immunity.
in vitro IL-4 neutralization
Ueda, A., et al (2012). "Fyn promotes Th17 differentiation by regulating the kinetics of RORgammat and Foxp3 expression" J Immunol 188(11): 5247-5256.
PubMed
Th17 cells constitute a proinflammatory CD4(+) T cell subset that is important for microbial clearance, but also are implicated as propagators of various autoimmune pathologies. Evidence suggests that Th17 cells share common progenitors with immunosuppressive CD4(+) inducible regulatory T cells (T(REG)) and that the developmental pathways of these two subsets are reciprocally regulated. In this study, we show evidence that the Src family tyrosine kinase Fyn helps regulate this Th17/T(REG) balance. When placed under Th17-skewing conditions, CD4(+) T cells from fyn(-/-) mice had decreased levels of IL-17, but increased expression of the T(REG) transcription factor Foxp3. The defect in IL-17 expression occurred independently of the ectopic Foxp3 expression and correlated with a delay in retinoic acid-related orphan receptor gammat upregulation and an inability to maintain normal STAT3 activation. Fyn-deficient Th17 cells also exhibited delayed upregulation of Il23r, Il21, Rora, and Irf4, as well as aberrant expression of Socs3, suggesting that Fyn may function upstream of a variety of molecular pathways that contribute to Th17 polarization. The fyn(-/-) mice had fewer IL-17(+)CD4(+) T cells in the large intestinal lamina propria compared with littermate controls. Furthermore, after transfer of either wild-type or fyn(-/-) naive CD4(+) T cells into Rag1(-/-) hosts, recipients receiving fyn(-/-) cells had fewer IL-17-producing T cells, indicating that Fyn may also regulate Th17 differentiation in vivo. These results identify Fyn as a possible novel regulator of the developmental balance between the Th17 cell and T(REG) subsets.
in vitro IL-4 neutralization
Li, X., et al (2012). "Divergent requirement for Galphas and cAMP in the differentiation and inflammatory profile of distinct mouse Th subsets" J Clin Invest 122(3): 963-973.
PubMed
cAMP, the intracellular signaling molecule produced in response to GPCR signaling, has long been recognized as an immunosuppressive agent that inhibits T cell receptor activation and T cell function. However, recent studies show that cAMP also promotes T cell-mediated immunity. Central to cAMP production downstream of GPCR activation is the trimeric G protein Gs. In order to reconcile the reports of divergent effects of cAMP in T cells and to define the direct effect of cAMP in T cells, we engineered mice in which the stimulatory Galpha subunit of Gs (Galphas) could be deleted in T cells using CD4-Cre (Gnas(DeltaCD4)). Gnas(DeltaCD4) CD4(+) T cells had reduced cAMP accumulation and Ca2(+) influx. In vitro and in vivo, Gnas(DeltaCD4) CD4(+) T cells displayed impaired differentiation to specific Th subsets: Th17 and Th1 cells were reduced or absent, but Th2 and regulatory T cells were unaffected. Furthermore, Gnas(DeltaCD4) CD4(+) T cells failed to provoke colitis in an adoptive transfer model, indicating reduced inflammatory function. Restoration of cAMP levels rescued the impaired phenotype of Gnas(DeltaCD4) CD4(+) T cells, reinstated the PKA-dependent influx of Ca2(+), and enhanced the ability of these cells to induce colitis. Our findings thus define an important role for cAMP in the differentiation of Th subsets and their subsequent inflammatory responses, and provide evidence that altering cAMP levels in CD4(+) T cells could provide an immunomodulatory approach targeting specific Th subsets.
in vivo IL-4 neutralization
in vivo blocking of IL-10/IL-10R signaling
Mishra, P. K., et al (2013). "Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response" Mucosal Immunol 6(2): 297-308.
PubMed
Helminth infection can prevent type 1 diabetes (T1D); however, the regulatory mechanisms inhibiting disease remain largely undefined. In these studies, nonobese diabetic (NOD) IL-4(-/-) mice were infected with the strictly enteric nematode parasite, Heligmosomoides polygyrus. Short-term infection, 5-7 weeks of age, inhibited T1D onset, as late as 40 weeks of age. CD4(+) T-cell STAT6 phosphorylation was inhibited, while suppressed signal transducer and activator of transcription 1 phosphorylation was sustained, as were increases in FOXP3(-), CD4(+) T-cell interleukin (IL)-10 production. Blockade of IL-10 signaling in NOD-IL-4(-/-), but not in NOD, mice during this short interval abrogated protective effects resulting in pancreatic beta-cell destruction and ultimately T1D. Transfer of CD4(+) T cells from H. polygyrus (Hp)-inoculated NOD IL-4(-/-) mice to NOD mice blocked the onset of T1D. These studies indicate that Hp infection induces non-T-regulatory cells to produce IL-10 independently of STAT6 signaling and that in this Th2-deficient environment IL-10 is essential for T1D inhibition.
McKinstry, K. K., et al (2014). "Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2" Nat Commun 5: 5377.
PubMed
It is unclear how CD4 T-cell memory formation is regulated following pathogen challenge, and when critical mechanisms act to determine effector T-cell fate. Here, we report that following influenza infection most effectors require signals from major histocompatibility complex class II molecules and CD70 during a late window well after initial priming to become memory. During this timeframe, effector cells must produce IL-2 or be exposed to high levels of paracrine or exogenously added IL-2 to survive an otherwise rapid default contraction phase. Late IL-2 promotes survival through acute downregulation of apoptotic pathways in effector T cells and by permanently upregulating their IL-7 receptor expression, enabling IL-7 to sustain them as memory T cells. This new paradigm defines a late checkpoint during the effector phase at which cognate interactions direct CD4 T-cell memory generation.
in vitro IL-4 neutralization
in vitro T cell stimulation/activation
Heinemann, C., et al (2014). "IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1" Nat Commun 5: 3770.
PubMed
Central nervous system (CNS) autoimmunity is regulated by the balance of pro-inflammatory cytokines and IL-10. Here we identify the transcriptional regulator Blimp1 as crucial to induce IL-10 in inflammatory T helper cells. Pre-committed Th17 cells respond to IL-27 and IL-12 by upregulating Blimp1 and adopt a Tr-1-like phenotype characterized by IL-10 and IFN-gamma production. Accordingly, Blimp1-deficient effector T cells fail to produce IL-10, and deficiency in Tr-1 cell function leads to uncontrolled Th17 cell-driven CNS pathology without the need to stabilize the Th17 phenotype with IL-23. IL-23 counteracts IL-27 and IL-12-mediated effects on Tr-1-development reinforcing the pro-inflammatory phenotype of Th17 cells. Thus, the balance of IL-23 vs IL-12/IL-27 signals into CD4(+) effector T cells determines whether tissue inflammation is perpetuated or resolves.
in vitro IL-4 neutralization
in vitro T cell stimulation/activation
in vitro T cell stimulation/activation
Hill, E. V., et al (2015). "Glycogen synthase kinase-3 controls IL-10 expression in CD4(+) effector T-cell subsets through epigenetic modification of the IL-10 promoter" Eur J Immunol 45(4): 1103-1115.
PubMed
The serine/threonine kinase glycogen synthase kinase-3 (GSK3) plays an important role in balancing pro- and anti-inflammatory cytokines. We have examined the role of GSK3 in production of IL-10 by subsets of CD4(+) T helper cells. Treatment of naive murine CD4(+) T cells with GSK3 inhibitors did not affect their production of IL-10. However, treatment of Th1 and Th2 cells with GSK3 inhibitors dramatically increased production of IL-10. GSK3 inhibition also led to upregulation of IL-10 among Th1, Th2, and Th17 subsets isolated from human blood. The encephalitogenic potential of GSK3 inhibitor treated murine Th1 cells was significantly reduced in adoptive transfer experiments by an IL-10-dependent mechanism. Analysis of the murine IL-10 promoter in response to inhibition of GSK3 in Th1 cells showed modification to a transcriptionally active state indicated by changes in histone H3 acetylation and methylation. Additionally, GSK3 inhibition increased expression of the transcription factors c-Maf, Nfil3, and GATA3, correlating with the increase in IL-10. These findings are important in the context of autoimmune disease since they show that it is possible to reprogram disease-causing cells through GSK3 inhibition.
in vitro IFNγ neutralization
Flow Cytometry
in vitro IL-4 neutralization
Hou, L., et al (2015). "The protease cathepsin L regulates Th17 cell differentiation" J Autoimmun. S 0896-8411(15): 30024-X.
PubMed
Previously we reported that IL-17+ T cells, primarily IL-17+ gammadelta cells, are increased in mice lacking the protease inhibitor serpinB1 (serpinb1-/- mice). Here we show that serpinB1-deficient CD4 cells exhibit a cell-autonomous and selective deficiency in suppressing T helper 17 (Th17) cell differentiation. This suggested an opposing role for one or more protease in promoting Th17 differentiation. We found that several SerpinB1-inhibitable cysteine cathepsins are induced in Th17 cells, most prominently cathepsin L (catL); this was verified by peptidase assays, active site labeling and Western blots. Moreover, Th17 differentiation was suppressed by both broad cathepsin inhibitors and catL selective inhibitors. CatL is present in Th17 cells as single chain (SC)- and two-chain (TC)-forms. Inhibiting asparagine endopeptidase (AEP) blocked conversion of SC-catL to TC-catL and increased generation of serpinb1-/- Th17 cells, but not wild-type Th17 cells. These findings suggest that SC-catL is biologically active in promoting Th17 generation and is counter-regulated by serpinB1 and secondarily by AEP. Thus, in addition to regulation by cytokines and transcription factors, differentiation of CD4 cells to Th17 cells is actively regulated by a catL-serpinB1-AEP module. Targeting this protease regulatory module could be an approach to treating Th17 cell-driven autoimmune disorders.
in vitro IFNγ neutralization
in vitro IL-4 neutralization
in vitro TGFβ neutralization
in vitro IL-12 neutralization
in vitro T cell stimulation/activation
in vitro T cell stimulation/activation
Choi, Y. S., et al (2015). "LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6" Nat Immunol 16(9): 980-990.
PubMed
Follicular helper T cells (TFH cells) are specialized effector CD4(+) T cells that help B cells develop germinal centers (GCs) and memory. However, the transcription factors that regulate the differentiation of TFH cells remain incompletely understood. Here we report that selective loss of Lef1 or Tcf7 (which encode the transcription factor LEF-1 or TCF-1, respectively) resulted in TFH cell defects, while deletion of both Lef1 and Tcf7 severely impaired the differentiation of TFH cells and the formation of GCs. Forced expression of LEF-1 enhanced TFH differentiation. LEF-1 and TCF-1 coordinated such differentiation by two general mechanisms. First, they established the responsiveness of naive CD4(+) T cells to TFH cell signals. Second, they promoted early TFH differentiation via the multipronged approach of sustaining expression of the cytokine receptors IL-6Ralpha and gp130, enhancing expression of the costimulatory receptor ICOS and promoting expression of the transcriptional repressor Bcl6.
in vitro IFNγ neutralization
in vitro IL-4 neutralization
Clever, D., et al (2016). "Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche" Cell 166(5): 1117-1131 e1114.
PubMed
Cancer cells must evade immune responses at distant sites to establish metastases. The lung is a frequent site for metastasis. We hypothesized that lung-specific immunoregulatory mechanisms create an immunologically permissive environment for tumor colonization. We found that T-cell-intrinsic expression of the oxygen-sensing prolyl-hydroxylase (PHD) proteins is required to maintain local tolerance against innocuous antigens in the lung but powerfully licenses colonization by circulating tumor cells. PHD proteins limit pulmonary type helper (Th)-1 responses, promote CD4(+)-regulatory T (Treg) cell induction, and restrain CD8(+) T cell effector function. Tumor colonization is accompanied by PHD-protein-dependent induction of pulmonary Treg cells and suppression of IFN-gamma-dependent tumor clearance. T-cell-intrinsic deletion or pharmacological inhibition of PHD proteins limits tumor colonization of the lung and improves the efficacy of adoptive cell transfer immunotherapy. Collectively, PHD proteins function in T cells to coordinate distinct immunoregulatory programs within the lung that are permissive to cancer metastasis.
in vivo IL-4 neutralization
in vivo IL-4 receptor stimulation (as a complex with IL-4)
Gaya, M., et al (2018). "Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells" Cell 172(3): 517-533 e520.
PubMed
B cells constitute an essential line of defense from pathogenic infections through the generation of class-switched antibody-secreting cells (ASCs) in germinal centers. Although this process is known to be regulated by follicular helper T (TfH) cells, the mechanism by which B cells initially seed germinal center reactions remains elusive. We found that NKT cells, a population of innate-like T lymphocytes, are critical for the induction of B cell immunity upon viral infection. The positioning of NKT cells at the interfollicular areas of lymph nodes facilitates both their direct priming by resident macrophages and the localized delivery of innate signals to antigen-experienced B cells. Indeed, NKT cells secrete an early wave of IL-4 and constitute up to 70% of the total IL-4-producing cells during the initial stages of infection. Importantly, the requirement of this innate immunity arm appears to be evolutionarily conserved because early NKT and IL-4 gene signatures also positively correlate with the levels of neutralizing antibodies in Zika-virus-infected macaques. In conclusion, our data support a model wherein a pre-TfH wave of IL-4 secreted by interfollicular NKT cells triggers the seeding of germinal center cells and serves as an innate link between viral infection and B cell immunity.
in vitro T cell stimulation/activation
Immunofluorescence
in vitro IL-4 neutralization
Kim, Y. U., et al (2015). "Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells" PLoS One 10(3): e0120294.
PubMed
BXD2 mice spontaneously develop autoantibodies and subsequent glomerulonephritis, offering a useful animal model to study autoimmune lupus. Although initial studies showed a critical contribution of IL-17 and Th17 cells in mediating autoimmune B cell responses in BXD2 mice, the role of follicular helper T (Tfh) cells remains incompletely understood. We found that both the frequency of Th17 cells and the levels of IL-17 in circulation in BXD2 mice were comparable to those of wild-type. By contrast, the frequency of PD-1+ CXCR5+ Tfh cells was significantly increased in BXD2 mice compared with wild-type mice, while the frequency of PD-1+ CXCR5+ Foxp3+ follicular regulatory T (Tfr) cells was reduced in the former group. The frequency of Tfh cells rather than that of Th17 cells was positively correlated with the frequency of germinal center B cells as well as the levels of autoantibodies to dsDNA. More importantly, CXCR5+ CD4+ T cells isolated from BXD2 mice induced the production of IgG from naive B cells in an IL-21-dependent manner, while CCR6+ CD4+ T cells failed to do so. These results together demonstrate that Tfh cells rather than Th17 cells contribute to the autoimmune germinal center reactions in BXD2 mice.
Flow Cytometry
in vitro IFNγ neutralization
Flow Cytometry
in vitro T cell stimulation/activation
in vitro IL-4 neutralization
Flow Cytometry
in vitro T cell stimulation/activation
Gu, A. D., et al (2015). "A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor beta receptor signaling" Immunity 42(1): 68-79.
PubMed
Transforming growth factor-beta (TGF-beta) suppresses T cell function to maintain self-tolerance and to promote tumor immune evasion. Yet how Smad4, a transcription factor component of TGF-beta signaling, regulates T cell function remains unclear. Here we have demonstrated an essential role for Smad4 in promoting T cell function during autoimmunity and anti-tumor immunity. Smad4 deletion rescued the lethal autoimmunity resulting from transforming growth factor-beta receptor (TGF-betaR) deletion and compromised T-cell-mediated tumor rejection. Although Smad4 was dispensable for T cell generation, homeostasis, and effector function, it was essential for T cell proliferation after activation in vitro and in vivo. The transcription factor Myc was identified to mediate Smad4-controlled T cell proliferation. This study thus reveals a requirement of Smad4 for T-cell-mediated autoimmunity and tumor rejection, which is beyond the current paradigm. It highlights a TGF-betaR-independent role for Smad4 in promoting T cell function, autoimmunity, and anti-tumor immunity.
in vivo IL-4 neutralization
in vivo IL-4 receptor stimulation (as a complex with IL-4)
Gaya, M., et al (2018). "Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells" Cell 172(3): 517-533 e520.
PubMed
B cells constitute an essential line of defense from pathogenic infections through the generation of class-switched antibody-secreting cells (ASCs) in germinal centers. Although this process is known to be regulated by follicular helper T (TfH) cells, the mechanism by which B cells initially seed germinal center reactions remains elusive. We found that NKT cells, a population of innate-like T lymphocytes, are critical for the induction of B cell immunity upon viral infection. The positioning of NKT cells at the interfollicular areas of lymph nodes facilitates both their direct priming by resident macrophages and the localized delivery of innate signals to antigen-experienced B cells. Indeed, NKT cells secrete an early wave of IL-4 and constitute up to 70% of the total IL-4-producing cells during the initial stages of infection. Importantly, the requirement of this innate immunity arm appears to be evolutionarily conserved because early NKT and IL-4 gene signatures also positively correlate with the levels of neutralizing antibodies in Zika-virus-infected macaques. In conclusion, our data support a model wherein a pre-TfH wave of IL-4 secreted by interfollicular NKT cells triggers the seeding of germinal center cells and serves as an innate link between viral infection and B cell immunity.
in vitro IL-4 neutralization
Clever, D., et al (2016). "Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche" Cell 166(5): 1117-1131 e1114.
PubMed
Cancer cells must evade immune responses at distant sites to establish metastases. The lung is a frequent site for metastasis. We hypothesized that lung-specific immunoregulatory mechanisms create an immunologically permissive environment for tumor colonization. We found that T-cell-intrinsic expression of the oxygen-sensing prolyl-hydroxylase (PHD) proteins is required to maintain local tolerance against innocuous antigens in the lung but powerfully licenses colonization by circulating tumor cells. PHD proteins limit pulmonary type helper (Th)-1 responses, promote CD4(+)-regulatory T (Treg) cell induction, and restrain CD8(+) T cell effector function. Tumor colonization is accompanied by PHD-protein-dependent induction of pulmonary Treg cells and suppression of IFN-gamma-dependent tumor clearance. T-cell-intrinsic deletion or pharmacological inhibition of PHD proteins limits tumor colonization of the lung and improves the efficacy of adoptive cell transfer immunotherapy. Collectively, PHD proteins function in T cells to coordinate distinct immunoregulatory programs within the lung that are permissive to cancer metastasis.
in vitro IL-4 neutralization
Flow Cytometry
Gu, A. D., et al (2015). "A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor beta receptor signaling" Immunity 42(1): 68-79.
PubMed
Transforming growth factor-beta (TGF-beta) suppresses T cell function to maintain self-tolerance and to promote tumor immune evasion. Yet how Smad4, a transcription factor component of TGF-beta signaling, regulates T cell function remains unclear. Here we have demonstrated an essential role for Smad4 in promoting T cell function during autoimmunity and anti-tumor immunity. Smad4 deletion rescued the lethal autoimmunity resulting from transforming growth factor-beta receptor (TGF-betaR) deletion and compromised T-cell-mediated tumor rejection. Although Smad4 was dispensable for T cell generation, homeostasis, and effector function, it was essential for T cell proliferation after activation in vitro and in vivo. The transcription factor Myc was identified to mediate Smad4-controlled T cell proliferation. This study thus reveals a requirement of Smad4 for T-cell-mediated autoimmunity and tumor rejection, which is beyond the current paradigm. It highlights a TGF-betaR-independent role for Smad4 in promoting T cell function, autoimmunity, and anti-tumor immunity.
in vitro IL-4 neutralization
Kim, Y. U., et al (2015). "Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells" PLoS One 10(3): e0120294.
PubMed
BXD2 mice spontaneously develop autoantibodies and subsequent glomerulonephritis, offering a useful animal model to study autoimmune lupus. Although initial studies showed a critical contribution of IL-17 and Th17 cells in mediating autoimmune B cell responses in BXD2 mice, the role of follicular helper T (Tfh) cells remains incompletely understood. We found that both the frequency of Th17 cells and the levels of IL-17 in circulation in BXD2 mice were comparable to those of wild-type. By contrast, the frequency of PD-1+ CXCR5+ Tfh cells was significantly increased in BXD2 mice compared with wild-type mice, while the frequency of PD-1+ CXCR5+ Foxp3+ follicular regulatory T (Tfr) cells was reduced in the former group. The frequency of Tfh cells rather than that of Th17 cells was positively correlated with the frequency of germinal center B cells as well as the levels of autoantibodies to dsDNA. More importantly, CXCR5+ CD4+ T cells isolated from BXD2 mice induced the production of IgG from naive B cells in an IL-21-dependent manner, while CCR6+ CD4+ T cells failed to do so. These results together demonstrate that Tfh cells rather than Th17 cells contribute to the autoimmune germinal center reactions in BXD2 mice.
in vitro IL-4 neutralization
Hou, L., et al (2015). "The protease cathepsin L regulates Th17 cell differentiation" J Autoimmun. S 0896-8411(15): 30024-X.
PubMed
Previously we reported that IL-17+ T cells, primarily IL-17+ gammadelta cells, are increased in mice lacking the protease inhibitor serpinB1 (serpinb1-/- mice). Here we show that serpinB1-deficient CD4 cells exhibit a cell-autonomous and selective deficiency in suppressing T helper 17 (Th17) cell differentiation. This suggested an opposing role for one or more protease in promoting Th17 differentiation. We found that several SerpinB1-inhibitable cysteine cathepsins are induced in Th17 cells, most prominently cathepsin L (catL); this was verified by peptidase assays, active site labeling and Western blots. Moreover, Th17 differentiation was suppressed by both broad cathepsin inhibitors and catL selective inhibitors. CatL is present in Th17 cells as single chain (SC)- and two-chain (TC)-forms. Inhibiting asparagine endopeptidase (AEP) blocked conversion of SC-catL to TC-catL and increased generation of serpinb1-/- Th17 cells, but not wild-type Th17 cells. These findings suggest that SC-catL is biologically active in promoting Th17 generation and is counter-regulated by serpinB1 and secondarily by AEP. Thus, in addition to regulation by cytokines and transcription factors, differentiation of CD4 cells to Th17 cells is actively regulated by a catL-serpinB1-AEP module. Targeting this protease regulatory module could be an approach to treating Th17 cell-driven autoimmune disorders.
in vitro IL-4 neutralization
Hill, E. V., et al (2015). "Glycogen synthase kinase-3 controls IL-10 expression in CD4(+) effector T-cell subsets through epigenetic modification of the IL-10 promoter" Eur J Immunol 45(4): 1103-1115.
PubMed
The serine/threonine kinase glycogen synthase kinase-3 (GSK3) plays an important role in balancing pro- and anti-inflammatory cytokines. We have examined the role of GSK3 in production of IL-10 by subsets of CD4(+) T helper cells. Treatment of naive murine CD4(+) T cells with GSK3 inhibitors did not affect their production of IL-10. However, treatment of Th1 and Th2 cells with GSK3 inhibitors dramatically increased production of IL-10. GSK3 inhibition also led to upregulation of IL-10 among Th1, Th2, and Th17 subsets isolated from human blood. The encephalitogenic potential of GSK3 inhibitor treated murine Th1 cells was significantly reduced in adoptive transfer experiments by an IL-10-dependent mechanism. Analysis of the murine IL-10 promoter in response to inhibition of GSK3 in Th1 cells showed modification to a transcriptionally active state indicated by changes in histone H3 acetylation and methylation. Additionally, GSK3 inhibition increased expression of the transcription factors c-Maf, Nfil3, and GATA3, correlating with the increase in IL-10. These findings are important in the context of autoimmune disease since they show that it is possible to reprogram disease-causing cells through GSK3 inhibition.
in vitro IL-4 neutralization
Choi, Y. S., et al (2015). "LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6" Nat Immunol 16(9): 980-990.
PubMed
Follicular helper T cells (TFH cells) are specialized effector CD4(+) T cells that help B cells develop germinal centers (GCs) and memory. However, the transcription factors that regulate the differentiation of TFH cells remain incompletely understood. Here we report that selective loss of Lef1 or Tcf7 (which encode the transcription factor LEF-1 or TCF-1, respectively) resulted in TFH cell defects, while deletion of both Lef1 and Tcf7 severely impaired the differentiation of TFH cells and the formation of GCs. Forced expression of LEF-1 enhanced TFH differentiation. LEF-1 and TCF-1 coordinated such differentiation by two general mechanisms. First, they established the responsiveness of naive CD4(+) T cells to TFH cell signals. Second, they promoted early TFH differentiation via the multipronged approach of sustaining expression of the cytokine receptors IL-6Ralpha and gp130, enhancing expression of the costimulatory receptor ICOS and promoting expression of the transcriptional repressor Bcl6.
in vitro IL-4 neutralization
Tang, W., et al (2014). "The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells" Immunity 41(4): 555-566.
PubMed
Bcl-3 is an atypical member of the IkappaB family that modulates transcription in the nucleus via association with p50 (NF-kappaB1) or p52 (NF-kappaB2) homodimers. Despite evidence attesting to the overall physiologic importance of Bcl-3, little is known about its cell-specific functions or mechanisms. Here we demonstrate a T-cell-intrinsic function of Bcl-3 in autoimmunity. Bcl-3-deficient T cells failed to induce disease in T cell transfer-induced colitis and experimental autoimmune encephalomyelitis. The protection against disease correlated with a decrease in Th1 cells that produced the cytokines IFN-gamma and GM-CSF and an increase in Th17 cells. Although differentiation into Th1 cells was not impaired in the absence of Bcl-3, differentiated Th1 cells converted to less-pathogenic Th17-like cells, in part via mechanisms involving expression of the RORgammat transcription factor. Thus, Bcl-3 constrained Th1 cell plasticity and promoted pathogenicity by blocking conversion to Th17-like cells, revealing a unique type of regulation that shapes adaptive immunity.
in vitro IL-4 neutralization
Burton, B. R., et al (2014). "Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy" Nat Commun 5: 4741.
PubMed
Antigen-specific immunotherapy combats autoimmunity or allergy by reinstating immunological tolerance to target antigens without compromising immune function. Optimization of dosing strategy is critical for effective modulation of pathogenic CD4(+) T-cell activity. Here we report that dose escalation is imperative for safe, subcutaneous delivery of the high self-antigen doses required for effective tolerance induction and elicits anergic, interleukin (IL)-10-secreting regulatory CD4(+) T cells. Analysis of the CD4(+) T-cell transcriptome, at consecutive stages of escalating dose immunotherapy, reveals progressive suppression of transcripts positively regulating inflammatory effector function and repression of cell cycle pathways. We identify transcription factors, c-Maf and NFIL3, and negative co-stimulatory molecules, LAG-3, TIGIT, PD-1 and TIM-3, which characterize this regulatory CD4(+) T-cell population and whose expression correlates with the immunoregulatory cytokine IL-10. These results provide a rationale for dose escalation in T-cell-directed immunotherapy and reveal novel immunological and transcriptional signatures as surrogate markers of successful immunotherapy.
in vitro IL-4 neutralization
McKinstry, K. K., et al (2014). "Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2" Nat Commun 5: 5377.
PubMed
It is unclear how CD4 T-cell memory formation is regulated following pathogen challenge, and when critical mechanisms act to determine effector T-cell fate. Here, we report that following influenza infection most effectors require signals from major histocompatibility complex class II molecules and CD70 during a late window well after initial priming to become memory. During this timeframe, effector cells must produce IL-2 or be exposed to high levels of paracrine or exogenously added IL-2 to survive an otherwise rapid default contraction phase. Late IL-2 promotes survival through acute downregulation of apoptotic pathways in effector T cells and by permanently upregulating their IL-7 receptor expression, enabling IL-7 to sustain them as memory T cells. This new paradigm defines a late checkpoint during the effector phase at which cognate interactions direct CD4 T-cell memory generation.
in vivo IL-4 neutralization
Mishra, P. K., et al (2013). "Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response" Mucosal Immunol 6(2): 297-308.
PubMed
Helminth infection can prevent type 1 diabetes (T1D); however, the regulatory mechanisms inhibiting disease remain largely undefined. In these studies, nonobese diabetic (NOD) IL-4(-/-) mice were infected with the strictly enteric nematode parasite, Heligmosomoides polygyrus. Short-term infection, 5-7 weeks of age, inhibited T1D onset, as late as 40 weeks of age. CD4(+) T-cell STAT6 phosphorylation was inhibited, while suppressed signal transducer and activator of transcription 1 phosphorylation was sustained, as were increases in FOXP3(-), CD4(+) T-cell interleukin (IL)-10 production. Blockade of IL-10 signaling in NOD-IL-4(-/-), but not in NOD, mice during this short interval abrogated protective effects resulting in pancreatic beta-cell destruction and ultimately T1D. Transfer of CD4(+) T cells from H. polygyrus (Hp)-inoculated NOD IL-4(-/-) mice to NOD mice blocked the onset of T1D. These studies indicate that Hp infection induces non-T-regulatory cells to produce IL-10 independently of STAT6 signaling and that in this Th2-deficient environment IL-10 is essential for T1D inhibition.
in vitro IL-4 neutralization
Li, X., et al (2012). "Divergent requirement for Galphas and cAMP in the differentiation and inflammatory profile of distinct mouse Th subsets" J Clin Invest 122(3): 963-973.
PubMed
cAMP, the intracellular signaling molecule produced in response to GPCR signaling, has long been recognized as an immunosuppressive agent that inhibits T cell receptor activation and T cell function. However, recent studies show that cAMP also promotes T cell-mediated immunity. Central to cAMP production downstream of GPCR activation is the trimeric G protein Gs. In order to reconcile the reports of divergent effects of cAMP in T cells and to define the direct effect of cAMP in T cells, we engineered mice in which the stimulatory Galpha subunit of Gs (Galphas) could be deleted in T cells using CD4-Cre (Gnas(DeltaCD4)). Gnas(DeltaCD4) CD4(+) T cells had reduced cAMP accumulation and Ca2(+) influx. In vitro and in vivo, Gnas(DeltaCD4) CD4(+) T cells displayed impaired differentiation to specific Th subsets: Th17 and Th1 cells were reduced or absent, but Th2 and regulatory T cells were unaffected. Furthermore, Gnas(DeltaCD4) CD4(+) T cells failed to provoke colitis in an adoptive transfer model, indicating reduced inflammatory function. Restoration of cAMP levels rescued the impaired phenotype of Gnas(DeltaCD4) CD4(+) T cells, reinstated the PKA-dependent influx of Ca2(+), and enhanced the ability of these cells to induce colitis. Our findings thus define an important role for cAMP in the differentiation of Th subsets and their subsequent inflammatory responses, and provide evidence that altering cAMP levels in CD4(+) T cells could provide an immunomodulatory approach targeting specific Th subsets.
in vitro IL-4 neutralization
Ueda, A., et al (2012). "Fyn promotes Th17 differentiation by regulating the kinetics of RORgammat and Foxp3 expression" J Immunol 188(11): 5247-5256.
PubMed
Th17 cells constitute a proinflammatory CD4(+) T cell subset that is important for microbial clearance, but also are implicated as propagators of various autoimmune pathologies. Evidence suggests that Th17 cells share common progenitors with immunosuppressive CD4(+) inducible regulatory T cells (T(REG)) and that the developmental pathways of these two subsets are reciprocally regulated. In this study, we show evidence that the Src family tyrosine kinase Fyn helps regulate this Th17/T(REG) balance. When placed under Th17-skewing conditions, CD4(+) T cells from fyn(-/-) mice had decreased levels of IL-17, but increased expression of the T(REG) transcription factor Foxp3. The defect in IL-17 expression occurred independently of the ectopic Foxp3 expression and correlated with a delay in retinoic acid-related orphan receptor gammat upregulation and an inability to maintain normal STAT3 activation. Fyn-deficient Th17 cells also exhibited delayed upregulation of Il23r, Il21, Rora, and Irf4, as well as aberrant expression of Socs3, suggesting that Fyn may function upstream of a variety of molecular pathways that contribute to Th17 polarization. The fyn(-/-) mice had fewer IL-17(+)CD4(+) T cells in the large intestinal lamina propria compared with littermate controls. Furthermore, after transfer of either wild-type or fyn(-/-) naive CD4(+) T cells into Rag1(-/-) hosts, recipients receiving fyn(-/-) cells had fewer IL-17-producing T cells, indicating that Fyn may also regulate Th17 differentiation in vivo. These results identify Fyn as a possible novel regulator of the developmental balance between the Th17 cell and T(REG) subsets.