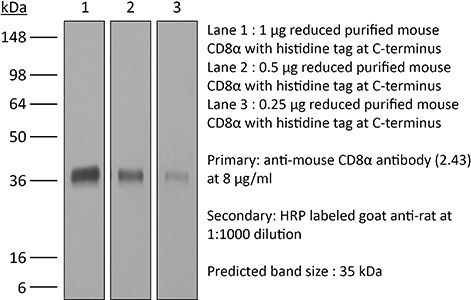
InVivoMAb anti-mouse CD8α
Product Details
The 2.43 monoclonal antibody reacts with mouse CD8α. The CD8 antigen is a transmembrane glycoprotein that acts as a co-receptor for the T cell receptor (TCR). Like the TCR, CD8 binds to class I MHC molecules displayed by antigen presenting cells (APC). CD8 is primarily expressed on the surface of cytotoxic T cells, but can also be found on thymocytes, natural killer cells, and some dendritic cell subsets. CD8 most commonly exists as a heterodimer composed of one CD8α and one CD8β chain however, it can also exist as a homodimer composed of two CD8α chains. Both the CD8α and CD8β chains share significant homology to immunoglobulin variable light chains. The molecular weight of each CD8 chain is approximately 34 kDa. The 2.43 antibody exhibits depleting activity when used in vivo.Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Immunogen | Mouse CTL clone L3 |
| Reported Applications |
in vivo CD8+ T cell depletion Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Sterility | 0.2 µm filtration |
| Production | Purified from tissue culture supernatant in an animal free facility |
| Purification | Protein G |
| RRID | AB_1125541 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
Flow Cytometry, in vivo CD8+ T cell depletion, in vivo CTLA-4 neutralization, in vivo IFNγ neutralization, in vivo MHC II blockade, in vivo TNFα neutralization
Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts PubMed
Adoptive transfer of large numbers of tumor-reactive CD8(+) cytotoxic T lymphocytes (CTLs) expanded and differentiated in vitro has shown promising clinical activity against cancer. However, such protocols are complicated by extensive ex vivo manipulations of tumor-reactive cells and have largely focused on CD8(+) CTLs, with much less emphasis on the role and contribution of CD4(+) T cells. Using a mouse model of advanced melanoma, we found that transfer of small numbers of naive tumor-reactive CD4(+) T cells into lymphopenic recipients induces substantial T cell expansion, differentiation, and regression of large established tumors without the need for in vitro manipulation. Surprisingly, CD4(+) T cells developed cytotoxic activity, and tumor rejection was dependent on class II-restricted recognition of tumors by tumor-reactive CD4(+) T cells. Furthermore, blockade of the coinhibitory receptor CTL-associated antigen 4 (CTLA-4) on the transferred CD4(+) T cells resulted in greater expansion of effector T cells, diminished accumulation of tumor-reactive regulatory T cells, and superior antitumor activity capable of inducing regression of spontaneous mouse melanoma. These findings suggest a novel potential therapeutic role for cytotoxic CD4(+) T cells and CTLA-4 blockade in cancer immunotherapy, and demonstrate the potential advantages of differentiating tumor-reactive CD4(+) cells in vivo over current protocols favoring in vitro expansion and differentiation.
in vivo CD8+ T cell depletion
The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel PubMed
PURPOSE: Interleukin-12 (IL12) is a potent proinflammatory cytokine with antitumor activity. Its heterodimeric nature makes it compatible with a large variety of different immunocytokine formats. Here we report the design, production, and characterization of a novel immunocytokine, based on the fusion of the F8 antibody (specific to the alternatively spliced EDA domain of fibronectin, a marker of tumor neovasculature) with IL12 (termed IL12-F8-F8). EXPERIMENTAL DESIGN: We developed a novel immunocytokine based on the sequential fusion of interleukin-12 as a single polypeptide with two F8 antibodies in single-chain Fv (scFv) format. The fusion protein was characterized in vitro, and its targeting performance was assessed in vivo. The immunocytokine antitumor activity was studied as monotherapy as well as in combination therapies in three different murine tumor models. Moreover, depletion experiments and tumor analysis revealed a dominant role of natural killer cells for the mechanism of action. RESULTS: IL12-F8-F8 can be produced in mammalian cells, yielding a product of good pharmaceutical quality, capable of selective localization on the tumor neovasculature in vivo, as judged by quantitative biodistribution analysis with radioiodinated protein preparations. The protein potently inhibited tumor growth in three different immunocompetent syngeneic models of cancer. The treatment was generally well tolerated. Moreover, the IL12-F8-F8 fusion protein could be produced both with murine IL12 (mIL12) and with human IL12 (hIL12). CONCLUSIONS: The potent antitumor activity of mIL12-F8-F8, studied alone or in combination with paclitaxel in different tumor models, paves the way to the clinical development of the fully human immunocytokine.
Flow Cytometry, in vivo CD8+ T cell depletion, in vivo NK cell depletion
Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma PubMed
Early phase clinical trials targeting the programmed death receptor-1/ligand-1 (PD-1/PD-L1) pathway to overcome tumor-mediated immunosuppression have reported promising results for a variety of cancers. This pathway appears to play an important role in the failure of immune reactivity to malignant plasma cells in multiple myeloma patients, as the tumor cells express relatively high levels of PD-L1, and T cells show increased PD-1 expression. In the current study, we demonstrate that PD-1/PD-L1 blockade with a PD-L1-specific Ab elicits rejection of a murine myeloma when combined with lymphodepleting irradiation. This particular combined approach by itself has not previously been shown to be efficacious in other tumor models. The antitumor effect of lymphodepletion/anti-PD-L1 therapy was most robust when tumor Ag-experienced T cells were present either through cell transfer or survival after nonmyeloablative irradiation. In vivo depletion of CD4 or CD8 T cells completely eliminated antitumor efficacy of the lymphodepletion/anti-PD-L1 therapy, indicating that both T cell subsets are necessary for tumor rejection. Elimination of myeloma by T cells occurs relatively quickly as tumor cells in the bone marrow were nearly nondetectable by 5 d after the first anti-PD-L1 treatment, suggesting that antimyeloma reactivity is primarily mediated by preactivated T cells, rather than newly generated myeloma-reactive T cells. Anti-PD-L1 plus lymphodepletion failed to improve survival in two solid tumor models, but demonstrated significant efficacy in two hematologic malignancy models. In summary, our results support the clinical testing of lymphodepletion and PD-1/PD-L1 blockade as a novel approach for improving the survival of patients with multiple myeloma.
In vivo CD103 neutralization, in vivo CD8+ T cell depletion
Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors PubMed
Although many human cancers are located in mucosal sites, most cancer vaccines are tested against subcutaneous tumors in preclinical models. We therefore wondered whether mucosa-specific homing instructions to the immune system might influence mucosal tumor outgrowth. We showed that the growth of orthotopic head and neck or lung cancers was inhibited when a cancer vaccine was delivered by the intranasal mucosal route but not the intramuscular route. This antitumor effect was dependent on CD8(+) T cells. Indeed, only intranasal vaccination elicited mucosal-specific CD8(+) T cells expressing the mucosal integrin CD49a. Blockade of CD49a decreased intratumoral CD8(+) T cell infiltration and the efficacy of cancer vaccine on mucosal tumor. We then showed that after intranasal vaccination, dendritic cells from lung parenchyma, but not those from spleen, induced the expression of CD49a on cocultured specific CD8(+) T cells. Tumor-infiltrating lymphocytes from human mucosal lung cancer also expressed CD49a, which supports the relevance and possible extrapolation of these results in humans. We thus identified a link between the route of vaccination and the induction of a mucosal homing program on induced CD8(+) T cells that controlled their trafficking. Immunization route directly affected the efficacy of the cancer vaccine to control mucosal tumors.
in vitro T cell stimulation/activation, in vivo CD8+ T cell depletion, in vivo IL-10 neutralization, in vivo IL-9 neutralization
The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells PubMed
The TH9 subset of helper T cells was initially shown to contribute to the induction of autoimmune and allergic diseases, but subsequent evidence has suggested that these cells also exert antitumor activities. However, the molecular events that account for their effector properties are elusive. Here we found that the transcription factor IRF1 enhanced the effector function of TH9 cells and dictated their anticancer properties. Under TH9-skewing conditions, interleukin 1beta (IL-1beta) induced phosphorylation of the transcription factor STAT1 and subsequent expression of IRF1, which bound to the promoters of Il9 and Il21 and enhanced secretion of the cytokines IL-9 and IL-21 from TH9 cells. Furthermore, IL-1beta-induced TH9 cells exerted potent anticancer functions in an IRF1- and IL-21-dependent manner. Our findings thus identify IRF1 as a target for controlling the function of TH9 cells.
in vivo CD8+ T cell depletion, in vivo TNFα neutralization
Soluble, but not transmembrane, TNF-alpha is required during influenza infection to limit the magnitude of immune responses and the extent of immunopathology PubMed
TNF-alpha is a pleotropic cytokine that has both proinflammatory and anti-inflammatory functions during influenza infection. TNF-alpha is first expressed as a transmembrane protein that is proteolytically processed to release a soluble form. Transmembrane TNF-alpha (memTNF-alpha) and soluble TNF-alpha (solTNF-alpha) have been shown to exert distinct tissue-protective or tissue-pathologic effects in several disease models. However, the relative contributions of memTNF-alpha or solTNF-alpha in regulating pulmonary immunopathology following influenza infection are unclear. Therefore, we performed intranasal influenza infection in mice exclusively expressing noncleavable memTNF-alpha or lacking TNF-alpha entirely and examined the outcomes. We found that solTNF-alpha, but not memTNF-alpha, was required to limit the size of the immune response and the extent of injury. In the absence of solTNF-alpha, there was a significant increase in the CD8(+) T cell response, including virus-specific CD8(+) T cells, which was due in part to an increased resistance to activation-induced cell death. We found that solTNF-alpha mediates these immunoregulatory effects primarily through TNFR1, because mice deficient in TNFR1, but not TNFR2, exhibited dysregulated immune responses and exacerbated injury similar to that observed in mice lacking solTNF-alpha. We also found that solTNF-alpha expression was required early during infection to regulate the magnitude of the CD8(+) T cell response, indicating that early inflammatory events are critical for the regulation of the effector phase. Taken together, these findings suggest that processing of memTNF-alpha to release solTNF-alpha is a critical event regulating the immune response during influenza infection.
ELISPOT, in vitro Fc receptor blocking, in vitro IFNγ neutralization, in vivo CD8+ T cell depletion, in vivo MDSC depletion, in vivo PD-L1 blockade, in vivo TNFα neutralization
Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice PubMed
High-dose ionizing irradiation (IR) results in direct tumor cell death and augments tumor-specific immunity, which enhances tumor control both locally and distantly. Unfortunately, local relapses often occur following IR treatment, indicating that IR-induced responses are inadequate to maintain antitumor immunity. Therapeutic blockade of the T cell negative regulator programmed death-ligand 1 (PD-L1, also called B7-H1) can enhance T cell effector function when PD-L1 is expressed in chronically inflamed tissues and tumors. Here, we demonstrate that PD-L1 was upregulated in the tumor microenvironment after IR. Administration of anti-PD-L1 enhanced the efficacy of IR through a cytotoxic T cell-dependent mechanism. Concomitant with IR-mediated tumor regression, we observed that IR and anti-PD-L1 synergistically reduced the local accumulation of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), which suppress T cells and alter the tumor immune microenvironment. Furthermore, activation of cytotoxic T cells with combination therapy mediated the reduction of MDSCs in tumors through the cytotoxic actions of TNF. Our data provide evidence for a close interaction between IR, T cells, and the PD-L1/PD-1 axis and establish a basis for the rational design of combination therapy with immune modulators and radiotherapy.
in vivo blocking of PD-1/PD-L signaling, in vivo CD8+ T cell depletion
Intratumoral administration of mRNA encoding a fusokine consisting of IFN-beta and the ectodomain of the TGF-beta receptor II potentiates antitumor immunity PubMed
It is generally accepted that the success of immunotherapy depends on the presence of tumor-specific CD8(+) cytotoxic T cells and the modulation of the tumor environment. In this study, we validated mRNA encoding soluble factors as a tool to modulate the tumor microenvironment to potentiate infiltration of tumor-specific T cells. Intratumoral delivery of mRNA encoding a fusion protein consisting of interferon-beta and the ectodomain of the transforming growth factor-beta receptor II, referred to as Fbeta(2), showed therapeutic potential. The treatment efficacy was dependent on CD8(+) T cells and could be improved through blockade of PD-1/PD-L1 interactions. In vitro studies revealed that administration of Fbeta(2) to tumor cells resulted in a reduced proliferation and increased expression of MHC I but also PD-L1. Importantly, Fbeta(2) enhanced the antigen presenting capacity of dendritic cells, whilst reducing the suppressive activity of myeloid-derived suppressor cells. In conclusion, these data suggest that intratumoral delivery of mRNA encoding soluble proteins, such as Fbeta(2), can modulate the tumor microenvironment, leading to effective antitumor T cell responses, which can be further potentiated through combination therapy.
in vivo CD8+ T cell depletion
VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors PubMed
Immune escape is a prerequisite for tumor development. To avoid the immune system, tumors develop different mechanisms, including T cell exhaustion, which is characterized by expression of immune inhibitory receptors, such as PD-1, CTLA-4, Tim-3, and a progressive loss of function. The recent development of therapies targeting PD-1 and CTLA-4 have raised great interest since they induced long-lasting objective responses in patients suffering from advanced metastatic tumors. However, the regulation of PD-1 expression, and thereby of exhaustion, is unclear. VEGF-A, a proangiogenic molecule produced by the tumors, plays a key role in the development of an immunosuppressive microenvironment. We report in the present work that VEGF-A produced in the tumor microenvironment enhances expression of PD-1 and other inhibitory checkpoints involved in CD8(+) T cell exhaustion, which could be reverted by anti-angiogenic agents targeting VEGF-A-VEGFR. In view of these results, association of anti-angiogenic molecules with immunomodulators of inhibitory checkpoints may be of particular interest in VEGF-A-producing tumors.
in vivo blocking of PD-1/PD-L signaling, in vivo CD4+ T cell depletion, in vivo CD8+ T cell depletion
TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity PubMed
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient’s individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFbeta activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFbeta neutralization during radiation therapy effectively generates CD8(+) T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNgamma and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFbeta blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFbeta blockade. Thus, TGFbeta is a fundamental regulator of radiation therapy’s ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFbeta neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
in vivo CD4+ T cell depletion, in vivo CD8+ T cell depletion, in vivo neutrophil depletion, in vivo NK cell depletion
Suppression of Fcgamma-receptor-mediated antibody effector function during persistent viral infection PubMed
Understanding how viruses subvert host immunity and persist is essential for developing strategies to eliminate infection. T cell exhaustion during chronic viral infection is well described, but effects on antibody-mediated effector activity are unclear. Herein, we show that increased amounts of immune complexes generated in mice persistently infected with lymphocytic choriomeningitis virus (LCMV) suppressed multiple Fcgamma-receptor (FcgammaR) functions. The high amounts of immune complexes suppressed antibody-mediated cell depletion, therapeutic antibody-killing of LCMV infected cells and human CD20-expressing tumors, as well as reduced immune complex-mediated cross-presentation to T cells. Suppression of FcgammaR activity was not due to inhibitory FcgammaRs or high concentrations of free antibody, and proper FcgammaR functions were restored when persistently infected mice specifically lacked immune complexes. Thus, we identify a mechanism of immunosuppression during viral persistence with implications for understanding effective antibody activity aimed at pathogen control.
in vivo blocking of PD-1/PD-L signaling, in vivo CD8+ T cell depletion, in vivo CTLA-4 neutralization, in vivo PD-L1 blockade
Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer PubMed
Immune checkpoint inhibitors result in impressive clinical responses, but optimal results will require combination with each other and other therapies. This raises fundamental questions about mechanisms of non-redundancy and resistance. Here we report major tumour regressions in a subset of patients with metastatic melanoma treated with an anti-CTLA4 antibody (anti-CTLA4) and radiation, and reproduced this effect in mouse models. Although combined treatment improved responses in irradiated and unirradiated tumours, resistance was common. Unbiased analyses of mice revealed that resistance was due to upregulation of PD-L1 on melanoma cells and associated with T-cell exhaustion. Accordingly, optimal response in melanoma and other cancer types requires radiation, anti-CTLA4 and anti-PD-L1/PD-1. Anti-CTLA4 predominantly inhibits T-regulatory cells (Treg cells), thereby increasing the CD8 T-cell to Treg (CD8/Treg) ratio. Radiation enhances the diversity of the T-cell receptor (TCR) repertoire of intratumoral T cells. Together, anti-CTLA4 promotes expansion of T cells, while radiation shapes the TCR repertoire of the expanded peripheral clones. Addition of PD-L1 blockade reverses T-cell exhaustion to mitigate depression in the CD8/Treg ratio and further encourages oligoclonal T-cell expansion. Similarly to results from mice, patients on our clinical trial with melanoma showing high PD-L1 did not respond to radiation plus anti-CTLA4, demonstrated persistent T-cell exhaustion, and rapidly progressed. Thus, PD-L1 on melanoma cells allows tumours to escape anti-CTLA4-based therapy, and the combination of radiation, anti-CTLA4 and anti-PD-L1 promotes response and immunity through distinct mechanisms.
Flow Cytometry, Immunohistochemistry (paraffin), in vivo CD8+ T cell depletion, in vivo IL-17A neutralization, in vivo IL-1β neutralization, in vivo IL-23p19 neutralization, in vivo neutrophil depletion
IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis PubMed
Metastatic disease remains the primary cause of death for patients with breast cancer. The different steps of the metastatic cascade rely on reciprocal interactions between cancer cells and their microenvironment. Within this local microenvironment and in distant organs, immune cells and their mediators are known to facilitate metastasis formation. However, the precise contribution of tumour-induced systemic inflammation to metastasis and the mechanisms regulating systemic inflammation are poorly understood. Here we show that tumours maximize their chance of metastasizing by evoking a systemic inflammatory cascade in mouse models of spontaneous breast cancer metastasis. We mechanistically demonstrate that interleukin (IL)-1beta elicits IL-17 expression from gamma delta (gammadelta) T cells, resulting in systemic, granulocyte colony-stimulating factor (G-CSF)-dependent expansion and polarization of neutrophils in mice bearing mammary tumours. Tumour-induced neutrophils acquire the ability to suppress cytotoxic T lymphocytes carrying the CD8 antigen, which limit the establishment of metastases. Neutralization of IL-17 or G-CSF and absence of gammadelta T cells prevents neutrophil accumulation and downregulates the T-cell-suppressive phenotype of neutrophils. Moreover, the absence of gammadelta T cells or neutrophils profoundly reduces pulmonary and lymph node metastases without influencing primary tumour progression. Our data indicate that targeting this novel cancer-cell-initiated domino effect within the immune system–the gammadelta T cell/IL-17/neutrophil axis–represents a new strategy to inhibit metastatic disease.
in vivo blocking of PD-1/PD-L signaling, in vivo CD8+ T cell depletion
Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
in vivo blocking of PD-1/PD-L signaling, in vivo CD4+ T cell depletion, in vivo CD8+ T cell depletion, in vivo eosinophil depletion, in vivo macrophage depletion, in vivo neutrophil depletion, in vivo NK cell depletion
Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
Flow Cytometry, in vivo CD4+ T cell depletion, in vivo CD8+ T cell depletion
Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses PubMed
The Macrophage Migration Inhibitory Factor (MIF) is an inflammatory cytokine that is overexpressed in a number of cancer types, with increased MIF expression often correlating with tumor aggressiveness and poor patient outcomes. In this study, we aimed to better understand the link between primary tumor expression of MIF and increased tumor growth. Using the MMTV-PyMT murine model of breast cancer, we observed that elevated MIF expression promoted tumor appearance and growth. Supporting this, we confirmed our previous observation that higher MIF expression supported tumor growth in the 4T1 murine model of breast cancer. We subsequently discovered that loss of MIF expression in 4T1 cells led to decreased cell numbers and increased apoptosis in vitro under reduced serum culture conditions. We hypothesized that this increase in cell death would promote detection by the host immune system in vivo, which could explain the observed impairment in tumor growth. Supporting this, we demonstrated that loss of MIF expression in the primary tumor led to an increased abundance of intra-tumoral IFNgamma-producing CD4+ and CD8+ T cells, and that depletion of T cells from mice bearing MIF-deficient tumors restored growth to the level of MIF-expressing tumors. Furthermore, we found that MIF depletion from the tumor cells resulted in greater numbers of activated intra-tumoral dendritic cells (DCs). Lastly, we demonstrated that loss of MIF expression led to a robust induction of a specialized form of cell death, immunogenic cell death (ICD), in vitro. Together, our data suggests a model in which MIF expression in the primary tumor dampens the anti-tumor immune response, promoting tumor growth.
in vivo blocking of PD-1/PD-L signaling, in vivo CD8+ T cell depletion
Co-inhibitory Molecule B7 Superfamily Member 1 Expressed by Tumor-Infiltrating Myeloid Cells Induces Dysfunction of Anti-tumor CD8(+) T Cells PubMed
The molecular mechanisms whereby CD8(+) T cells become “exhausted” in the tumor microenvironment remain unclear. Programmed death ligand-1 (PD-L1) is upregulated on tumor cells and PD-1-PD-L1 blockade has significant efficacy in human tumors; however, most patients do not respond, suggesting additional mechanisms underlying T cell exhaustion. B7 superfamily member 1 (B7S1), also called B7-H4, B7x, or VTCN1, negatively regulates T cell activation. Here we show increased B7S1 expression on myeloid cells from human hepatocellular carcinoma correlated with CD8(+) T cell dysfunction. B7S1 inhibition suppressed development of murine tumors. Putative B7S1 receptor was co-expressed with PD-1 but not T cell immunoglobulin and mucin-domain containing-3 (Tim-3) at an activated state of early tumor-infiltrating CD8(+) T cells, and B7S1 promoted T cell exhaustion, possibly through Eomes overexpression. Combinatorial blockade of B7S1 and PD-1 synergistically enhanced anti-tumor immune responses. Collectively, B7S1 initiates dysfunction of tumor-infiltrating CD8(+) T cells and may be targeted for cancer immunotherapy.
- Immunology and Microbiology,
- Cancer Research
Spon1+ inflammatory monocytes promote collagen remodeling and lung cancer metastasis through lipoprotein receptor 8 signaling.
In JCI Insight on 8 May 2024 by Whately, K. M., Sengottuvel, N., et al.
Lung cancer is the leading cause of cancer-related deaths in the world, and non-small cell lung cancer (NSCLC) is the most common subset. We previously found that infiltration of tumor inflammatory monocytes (TIMs) into lung squamous carcinoma (LUSC) tumors is associated with increased metastases and poor survival. To further understand how TIMs promote metastases, we compared RNA-Seq profiles of TIMs from several LUSC metastatic models with inflammatory monocytes (IMs) of non-tumor-bearing controls. We identified Spon1 as upregulated in TIMs and found that Spon1 expression in LUSC tumors corresponded with poor survival and enrichment of collagen extracellular matrix signatures. We observed SPON1+ TIMs mediate their effects directly through LRP8 on NSCLC cells, which resulted in TGF-β1 activation and robust production of fibrillar collagens. Using several orthogonal approaches, we demonstrated that SPON1+ TIMs were sufficient to promote NSCLC metastases. Additionally, we found that Spon1 loss in the host, or Lrp8 loss in cancer cells, resulted in a significant decrease of both high-density collagen matrices and metastases. Finally, we confirmed the relevance of the SPON1/LRP8/TGF-β1 axis with collagen production and survival in patients with NSCLC. Taken together, our study describes how SPON1+ TIMs promote collagen remodeling and NSCLC metastases through an LRP8/TGF-β1 signaling axis.
- Cancer Research,
- Immunology and Microbiology
Spermine Synthase Engages in Macrophages M2 polarization to Sabotage Antitumor Immunity in Hepatocellular Carcinoma
Preprint on Research Square on 19 March 2024 by Fang, Y., Sun, Y., et al.
Disturbances in tumor cell metabolism reshape the tumor microenvironment (TME) and impair antitumor immunity, but the implicit mechanisms remain elusive. Here, we found that spermine synthase (SMS) was significantly upregulated in tumor cells, which correlated positively with immunosuppressive microenvironments and predicted poor survival in hepatocellular carcinoma (HCC) patients. Via “subcutaneous” and “orthotopic” HCC syngeneic mouse models and a series of in vitro coculture experiments, we identified elevated SMS level in HCC cells played a role in immune escape mainly through its metabolic product spermine, which induced tumor-associated macrophage (TAM) reprogramming and subsequently corresponded with a decreased antitumor functionality of CD8 + T cells. Mechanistically, we discovered that spermine reprogrammed TAM mainly by activating the PI3K-Akt-mTOR-S6K signaling pathway. Spermine inhibition in combination with immune checkpoint blockade effectively diminishes tumor burden in vivo . Our results expand the understanding of the critical role of metabolites in regulating cancer progression and anti-tumor immunity, and open new avenues for developing novel therapeutic strategies against HCC.
- Immunology and Microbiology,
- Cancer Research
Interferon-γ in the tumor microenvironment promotes the expression of B7H4 in colorectal cancer cells, thereby inhibiting cytotoxic T cells.
In Scientific Reports on 13 March 2024 by Jing, Z. L., Liu, G. L., et al.
The bioactivity of interferon-γ (IFN-γ) in cancer cells in the tumor microenvironment (TME) is not well understood in the current immunotherapy era. We found that IFN-γ has an immunosuppressive effect on colorectal cancer (CRC) cells. The tumor volume in immunocompetent mice was significantly increased after subcutaneous implantation of murine CRC cells followed by IFN-γ stimulation, and RNA sequencing showed high expression of B7 homologous protein 4 (B7H4) in these tumors. B7H4 promotes CRC cell growth by inhibiting the release of granzyme B (GzmB) from CD8+ T cells and accelerating apoptosis in CD8+ T cells. Furthermore, interferon regulatory factor 1 (IRF1), which binds to the B7H4 promoter, is positively associated with IFN-γ stimulation-induced expression of B7H4. The clinical outcome of patients with CRC was negatively related to the high expression of B7H4 in cancer cells or low expression of CD8 in the microenvironment. Therefore, B7H4 is a biomarker of poor prognosis in CRC patients, and interference with the IFN-γ/IRF1/B7H4 axis might be a novel immunotherapeutic method to restore the cytotoxic killing of CRC cells. © 2024. The Author(s).
- Mus musculus (House mouse),
- Immunology and Microbiology,
- Cancer Research
Mi-2β promotes immune evasion in melanoma by activating EZH2 methylation.
In Nature Communications on 9 March 2024 by Li, C., Wang, Z., et al.
Recent development of new immune checkpoint inhibitors has been particularly successfully in cancer treatment, but still the majority patients fail to benefit. Converting resistant tumors to immunotherapy sensitive will provide a significant improvement in patient outcome. Here we identify Mi-2β as a key melanoma-intrinsic effector regulating the adaptive anti-tumor immune response. Studies in genetically engineered mouse melanoma models indicate that loss of Mi-2β rescues the immune response to immunotherapy in vivo. Mechanistically, ATAC-seq analysis shows that Mi-2β controls the accessibility of IFN-γ-stimulated genes (ISGs). Mi-2β binds to EZH2 and promotes K510 methylation of EZH2, subsequently activating the trimethylation of H3K27 to inhibit the transcription of ISGs. Finally, we develop an Mi-2β-targeted inhibitor, Z36-MP5, which reduces Mi-2β ATPase activity and reactivates ISG transcription. Consequently, Z36-MP5 induces a response to immune checkpoint inhibitors in otherwise resistant melanoma models. Our work provides a potential therapeutic strategy to convert immunotherapy resistant melanomas to sensitive ones. © 2024. The Author(s).
- Mus musculus (House mouse)
Ultra-low volume intradermal administration of radiation-attenuated sporozoites with the glycolipid adjuvant 7DW8-5 completely protects mice against malaria.
In Scientific Reports on 4 February 2024 by Watson, F. N., Shears, M. J., et al.
Radiation-attenuated sporozoite (RAS) vaccines can completely prevent blood stage Plasmodium infection by inducing liver-resident memory CD8+ T cells to target parasites in the liver. Such T cells can be induced by 'Prime-and-trap' vaccination, which here combines DNA priming against the P. yoelii circumsporozoite protein (CSP) with a subsequent intravenous (IV) dose of liver-homing RAS to "trap" the activated and expanding T cells in the liver. Prime-and-trap confers durable protection in mice, and efforts are underway to translate this vaccine strategy to the clinic. However, it is unclear whether the RAS trapping dose must be strictly administered by the IV route. Here we show that intradermal (ID) RAS administration can be as effective as IV administration if RAS are co-administrated with the glycolipid adjuvant 7DW8-5 in an ultra-low inoculation volume. In mice, the co-administration of RAS and 7DW8-5 in ultra-low ID volumes (2.5 µL) was completely protective and dose sparing compared to standard volumes (10-50 µL) and induced protective levels of CSP-specific CD8+ T cells in the liver. Our finding that adjuvants and ultra-low volumes are required for ID RAS efficacy may explain why prior reports about higher volumes of unadjuvanted ID RAS proved less effective than IV RAS. The ID route may offer significant translational advantages over the IV route and could improve sporozoite vaccine development. © 2024. The Author(s).
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Interplay between ATRX and IDH1 mutations governs innate immune responses in diffuse gliomas.
In Nature Communications on 25 January 2024 by Hariharan, S., Whitfield, B. T., et al.
Stimulating the innate immune system has been explored as a therapeutic option for the treatment of gliomas. Inactivating mutations in ATRX, defining molecular alterations in IDH-mutant astrocytomas, have been implicated in dysfunctional immune signaling. However, little is known about the interplay between ATRX loss and IDH mutation on innate immunity. To explore this, we generated ATRX-deficient glioma models in the presence and absence of the IDH1R132H mutation. ATRX-deficient glioma cells are sensitive to dsRNA-based innate immune agonism and exhibit impaired lethality and increased T-cell infiltration in vivo. However, the presence of IDH1R132H dampens baseline expression of key innate immune genes and cytokines in a manner restored by genetic and pharmacological IDH1R132H inhibition. IDH1R132H co-expression does not interfere with the ATRX deficiency-mediated sensitivity to dsRNA. Thus, ATRX loss primes cells for recognition of dsRNA, while IDH1R132H reversibly masks this priming. This work reveals innate immunity as a therapeutic vulnerability of astrocytomas. © 2024. The Author(s).
- Immunology and Microbiology,
- Cancer Research
Pharmaceutical targeting of OTUB2 sensitizes tumors to cytotoxic T cells via degradation of PD-L1.
In Nature Communications on 2 January 2024 by Ren, W., Xu, Z., et al.
PD-1 is a co-inhibitory receptor expressed by CD8+ T cells which limits their cytotoxicity. PD-L1 expression on cancer cells contributes to immune evasion by cancers, thus, understanding the mechanisms that regulate PD-L1 protein levels in cancers is important. Here we identify tumor-cell-expressed otubain-2 (OTUB2) as a negative regulator of antitumor immunity, acting through the PD-1/PD-L1 axis in various human cancers. Mechanistically, OTUB2 directly interacts with PD-L1 to disrupt the ubiquitination and degradation of PD-L1 in the endoplasmic reticulum. Genetic deletion of OTUB2 markedly decreases the expression of PD-L1 proteins on the tumor cell surface, resulting in increased tumor cell sensitivity to CD8+ T-cell-mediated cytotoxicity. To underscore relevance in human patients, we observe a significant correlation between OTUB2 expression and PD-L1 abundance in human non-small cell lung cancer. An inhibitor of OTUB2, interfering with its deubiquitinase activity without disrupting the OTUB2-PD-L1 interaction, successfully reduces PD-L1 expression in tumor cells and suppressed tumor growth. Together, these results reveal the roles of OTUB2 in PD-L1 regulation and tumor evasion and lays down the proof of principle for OTUB2 targeting as therapeutic strategy for cancer treatment. © 2024. The Author(s).
- Immunology and Microbiology,
- Cancer Research
Lactose blocks intercellular spreading of Galectin-1 from cancer cells to T-cells and activates tumor immunological control
Preprint on BioRxiv : the Preprint Server for Biology on 20 December 2023 by Hong, Y., Si, X., et al.
Understanding the mechanisms by which the immune system surveils cancer is the key to developing better tumor immunotherapy strategies. By CRISPR/Cas9 screenings, we identified that inactivation of beta-1,4-galactosyltransferase-1 (B4GALT1), a key enzyme in glycoconjugate biosynthesis, leads to enhanced T-cell receptor (TCR) activation and functions of CD8 + T-cells. Via proximity-dependent-intercellular-protein-spreading (PDICPS), cancer cells transfer surface-bound galectin-1 (Gal-1) proteins, which recognize and bind galactosylated membrane proteins, to CD8 + T-cells, thereby suppressing T-cell-mediated cytolysis. B4GALT1-deficiency leads to reduced cell-surface galactosylation and Gal-1 binding of CD8 + T-cells. Proteomic analysis revealed reduced binding of Gal-1 with TCR and its coreceptor CD8 on B4GALT1-deficient CD8 + T-cells, leading to enhanced TCR-CD8 colocalization and T-cell activation. Lactose, a structure-mimicking competitive inhibitor of N-glycan galactosylation, enhances the functions of CD8 + T-cells and tumor immunosurveillance. Results from various preclinical tumor models demonstrate that lactose and its derivatives are a new class of immune checkpoint inhibitors for tumor immunotherapy.
- Mus musculus (House mouse),
- Cancer Research
INHBA/Activin A promotes tumor growth and induces resistance to anti-PD-L1 therapy by suppressing IFN-γ signaling
Preprint on BioRxiv : the Preprint Server for Biology on 8 December 2023 by Li, F., Gu, L., et al.
Inhibin beta A (INHBA) and its homodimer activin A have pleiotropic effects on modulation of immune responses and tumor progression, respectively, but it remains uncertain whether tumors may release activin A to regulate anti-tumor immunity. As evidenced by our RNA-Seq and in vitro results, the interferon-γ (IFN-γ) signaling pathway was significantly down-regulated by tumor intrinsic activin A. Tumor INHBA deficiency led to lower expression of PD-L1 induced by IFN-γ, resulting in poor responsiveness to anti-PD-L1 therapy. On the other hand, decreased secretion of IFN-γ-stimulated chemokines, including C-X-C motif chemokine 9 (CXCL9) and 10 (CXCL10), impaired the infiltration of effector T cells into the tumor microenvironment. Furthermore, the activin A-specific antibody garetosmab improved anti-tumor immunity and its combination with the anti-PD-L1 antibody atezolizumab showed a superior therapeutic effect to monotherapy. Our findings reveal that INHBA/activin A is involved in anti-tumor immunity by inhibiting the IFN-γ signaling pathway and considered to be a potential target to overcome anti-PD-L1 resistance in clinical cancer treatment.
- Genetics,
- Immunology and Microbiology
Mice with FVB-derived sequence on chromosome 17 succumb to disseminated virus infection due to aberrant NK cell and T cell responses.
In IScience on 17 November 2023 by Tibbs, T. N., Donoghue, L. J., et al.
Zoonotic arenavirus infections can result in viral hemorrhagic disease, characterized by platelet loss, petechia, and multi-organ injury. The mechanisms governing these outcomes are likely impacted by virus strain and infection dose, as well as an individual's genetic background and immune constitution. To better understand the processes leading to severe pathogenesis, we compared two strains of inbred mice, C57BL/6J (B6) and FVB/NJ (FVB), that have diametrically opposed outcomes during disseminated lymphocytic choriomeningitis virus (LCMV) infection. Infection caused minimal pathogenesis in B6 mice, whereas FVB mice developed acute hepatitis and perished due, in part, to aberrant NK cell and T cell responses. Susceptible mice showed an outgrowth of cytolytic CD4+ T cells and loss of Treg cells. B6 congenic mice with the FVB allele at a 25Mb locus on chromosome 17 recapitulated FVB pathogenesis upon infection. A locus containing a limited number of variants in immune-related genes greatly impacts survival during infection. © 2023 The Author(s).
- Cancer Research,
- Immunology and Microbiology
Trans-vaccenic acid reprograms CD8+ T cells and anti-tumour immunity.
In Nature on 1 November 2023 by Guo, Y., Xia, S., et al.
Diet-derived nutrients are inextricably linked to human physiology by providing energy and biosynthetic building blocks and by functioning as regulatory molecules. However, the mechanisms by which circulating nutrients in the human body influence specific physiological processes remain largely unknown. Here we use a blood nutrient compound library-based screening approach to demonstrate that dietary trans-vaccenic acid (TVA) directly promotes effector CD8+ T cell function and anti-tumour immunity in vivo. TVA is the predominant form of trans-fatty acids enriched in human milk, but the human body cannot produce TVA endogenously1. Circulating TVA in humans is mainly from ruminant-derived foods including beef, lamb and dairy products such as milk and butter2,3, but only around 19% or 12% of dietary TVA is converted to rumenic acid by humans or mice, respectively4,5. Mechanistically, TVA inactivates the cell-surface receptor GPR43, an immunomodulatory G protein-coupled receptor activated by its short-chain fatty acid ligands6-8. TVA thus antagonizes the short-chain fatty acid agonists of GPR43, leading to activation of the cAMP-PKA-CREB axis for enhanced CD8+ T cell function. These findings reveal that diet-derived TVA represents a mechanism for host-extrinsic reprogramming of CD8+ T cells as opposed to the intrahost gut microbiota-derived short-chain fatty acids. TVA thus has translational potential for the treatment of tumours. © 2023. The Author(s).
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Targeted Inhibition of lncRNA Malat1 Alters the Tumor Immune Microenvironment in Preclinical Syngeneic Mouse Models of Triple-Negative Breast Cancer.
In Cancer Immunology Research on 1 November 2023 by Oluwatoyosi, A., Shen, Y., et al.
Long noncoding RNAs (lncRNA) play an important role in gene regulation in both normal tissues and cancer. Targeting lncRNAs is a promising therapeutic approach that has become feasible through the development of gapmer antisense oligonucleotides (ASO). Metastasis-associated lung adenocarcinoma transcript (Malat1) is an abundant lncRNA whose expression is upregulated in several cancers. Although Malat1 increases the migratory and invasive properties of tumor cells, its role in the tumor microenvironment (TME) is still not well defined. We explored the connection between Malat1 and the tumor immune microenvironment (TIME) using several immune-competent preclinical syngeneic Tp53-null triple-negative breast cancer (TNBC) mouse models that mimic the heterogeneity and immunosuppressive TME found in human breast cancer. Using a Malat1 ASO, we were able to knockdown Malat1 RNA expression resulting in a delay in primary tumor growth, decreased proliferation, and increased apoptosis. In addition, immunophenotyping of tumor-infiltrating lymphocytes revealed that Malat1 inhibition altered the TIME, with a decrease in immunosuppressive tumor-associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC) as well as an increase in cytotoxic CD8+ T cells. Malat1 depletion in tumor cells, TAMs, and MDSCs decreased immunosuppressive cytokine/chemokine secretion whereas Malat1 inhibition in T cells increased inflammatory secretions and T-cell proliferation. Combination of a Malat1 ASO with chemotherapy or immune checkpoint blockade (ICB) improved the treatment responses in a preclinical model. These studies highlight the immunostimulatory effects of Malat1 inhibition in TNBC, the benefit of a Malat1 ASO therapeutic, and its potential use in combination with chemotherapies and immunotherapies. ©2023 The Authors; Published by the American Association for Cancer Research.
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
BCL2 Inhibition Reveals a Dendritic Cell-Specific Immune Checkpoint That Controls Tumor Immunosurveillance.
In Cancer Discovery on 1 November 2023 by Zhao, L., Liu, P., et al.
We developed a phenotypic screening platform for the functional exploration of dendritic cells (DC). Here, we report a genome-wide CRISPR screen that revealed BCL2 as an endogenous inhibitor of DC function. Knockout of BCL2 enhanced DC antigen presentation and activation as well as the capacity of DCs to control tumors and to synergize with PD-1 blockade. The pharmacologic BCL2 inhibitors venetoclax and navitoclax phenocopied these effects and caused a cDC1-dependent regression of orthotopic lung cancers and fibrosarcomas. Thus, solid tumors failed to respond to BCL2 inhibition in mice constitutively devoid of cDC1, and this was reversed by the infusion of DCs. Moreover, cDC1 depletion reduced the therapeutic efficacy of BCL2 inhibitors alone or in combination with PD-1 blockade and treatment with venetoclax caused cDC1 activation, both in mice and in patients. In conclusion, genetic and pharmacologic BCL2 inhibition unveils a DC-specific immune checkpoint that restrains tumor immunosurveillance. BCL2 inhibition improves the capacity of DCs to stimulate anticancer immunity and restrain cancer growth in an immunocompetent context but not in mice lacking cDC1 or mature T cells. This study indicates that BCL2 blockade can be used to sensitize solid cancers to PD-1/PD-L1-targeting immunotherapy. This article is featured in Selected Articles from This Issue, p. 2293. ©2023 American Association for Cancer Research.
- Mus musculus (House mouse),
- Immunology and Microbiology,
- Cancer Research
Tumor Microenvironment Responsive CD8+ T Cells and Myeloid-Derived Suppressor Cells to Trigger CD73 Inhibitor AB680-Based Synergistic Therapy for Pancreatic Cancer.
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 November 2023 by Chen, Q., Yin, H., et al.
CD73 plays a critical role in the pathogenesis and immune escape in pancreatic ductal adenocarcinoma (PDAC). AB680, an exceptionally potent and selective inhibitor of CD73, is administered in an early clinical trial, in conjunction with gemcitabine and anti-PD-1 therapy, for the treatment of PDAC. Nevertheless, the specific therapeutic efficacy and immunoregulation within the microenvironment of AB680 monotherapy in PDAC have yet to be fully elucidated. In this study, AB680 exhibits a significant effect in augmenting the infiltration of responsive CD8+ T cells and prolongs the survival in both subcutaneous and orthotopic murine PDAC models. In parallel, it also facilitates chemotaxis of myeloid-derived suppressor cells (MDSCs) by tumor-derived CXCL5 in an AMP-dependent manner, which may potentially contribute to enhanced immunosuppression. The concurrent administration of AB680 and PD-1 blockade, rather than gemcitabine, synergistically restrain tumor growth. Notably, gemcitabine weakened the efficacy of AB680, which is dependent on CD8+ T cells. Finally, the supplementation of a CXCR2 inhibitor is validated to further enhance the therapeutic efficacy when combined with AB680 plus PD-1 inhibitor. These findings systematically demonstrate the efficacy and immunoregulatory mechanism of AB680, providing a novel, efficient, and promising immunotherapeutic combination strategy for PDAC. © 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Intermittent fasting induced ketogenesis inhibits mouse epithelial ovarian cancer by promoting antitumor T cell response.
In IScience on 20 October 2023 by Udumula, M. P., Singh, H., et al.
In various cancer models, dietary interventions have been shown to inhibit tumor growth, improve anticancer drug efficacy, and enhance immunity, but no such evidence exists for epithelial ovarian cancer (EOC), the most lethal gynecologic cancer. The anticancer immune responses induced by 16-h intermittent fasting (IF) were studied in mice with EOC. IF consistently reduced metabolic growth factors and cytokines that stimulate tumor growth, creating a tumor-hostile environment. Immune profiling showed that IF dramatically alters anti-cancer immunity by increasing CD4+ and CD8+ cells, Th1 and cytotoxic responses, and metabolic fitness. β-hydroxy butyrate (BHB), a bioactive metabolite produced by IF, partially imitates its anticancer effects by inducing CD8+ effector function. In a direct comparison, IF outperformed exogenous BHB treatment in survival and anti-tumor immune response, probably due to increased ketogenesis. Thus, IF and one of its metabolic mediators BHB suppress EOC growth and sustain a potent anti-tumor T cell response. © 2023 The Author(s).
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Targeting the pericyte antigen DLK1 with an alpha type-1 polarized dendritic cell vaccine results in tumor vascular modulation and protection against colon cancer progression.
In Frontiers in Immunology on 18 October 2023 by McCormick, A. L., Anderson, T. S., et al.
Despite the availability of various treatment options, colorectal cancer (CRC) remains a significant contributor to cancer-related mortality. Current standard-of-care interventions, including surgery, chemotherapy, and targeted agents like immune checkpoint blockade and anti-angiogenic therapies, have improved short-term patient outcomes depending on disease stage, but survival rates with metastasis remain low. A promising strategy to enhance the clinical experience with CRC involves the use of dendritic cell (DC) vaccines that incite immunity against tumor-derived blood vessels, which are necessary for CRC growth and progression. In this report, we target tumor-derived pericytes expressing DLK1 with a clinically-relevant alpha type-1 polarized DC vaccine (αDC1) in a syngeneic mouse model of colorectal cancer. Our pre-clinical data demonstrate the αDC1 vaccine's ability to induce anti-tumor effects by facilitating cytotoxic T lymphocyte activity and ablating the tumor vasculature. This work, overall, provides a foundation to further interrogate immune-mediated mechanisms of protection in order to help devise efficacious αDC1-based strategies for patients with CRC. Copyright © 2023 McCormick, Anderson, Daugherity, Okpalanwaka, Smith, Appiah and Lowe.
- Mus musculus (House mouse),
- Cancer Research,
- Endocrinology and Physiology,
- Immunology and Microbiology
Angiopoietin-2 blockade suppresses growth of liver metastases from pancreatic neuroendocrine tumors by promoting T cell recruitment.
In The Journal of Clinical Investigation on 16 October 2023 by Lee, E., O'Keefe, S., et al.
Improving the management of metastasis in pancreatic neuroendocrine tumors (PanNETs) is critical, as nearly half of patients with PanNETs present with liver metastases, and this accounts for the majority of patient mortality. We identified angiopoietin-2 (ANGPT2) as one of the most upregulated angiogenic factors in RNA-Seq data from human PanNET liver metastases and found that higher ANGPT2 expression correlated with poor survival rates. Immunohistochemical staining revealed that ANGPT2 was localized to the endothelial cells of blood vessels in PanNET liver metastases. We observed an association between the upregulation of endothelial ANGPT2 and liver metastatic progression in both patients and transgenic mouse models of PanNETs. In human and mouse PanNET liver metastases, ANGPT2 upregulation coincided with poor T cell infiltration, indicative of an immunosuppressive tumor microenvironment. Notably, both pharmacologic inhibition and genetic deletion of ANGPT2 in PanNET mouse models slowed the growth of PanNET liver metastases. Furthermore, pharmacologic inhibition of ANGPT2 promoted T cell infiltration and activation in liver metastases, improving the survival of mice with metastatic PanNETs. These changes were accompanied by reduced plasma leakage and improved vascular integrity in metastases. Together, these findings suggest that ANGPT2 blockade may be an effective strategy for promoting T cell infiltration and immunostimulatory reprogramming to reduce the growth of liver metastases in PanNETs.
- Mus musculus (House mouse),
- Biochemistry and Molecular biology,
- Immunology and Microbiology
mAb therapy controls CNS-resident lyssavirus infection via a CD4 T cell-dependent mechanism.
In EMBO Molecular Medicine on 11 October 2023 by Mastraccio, K. E., Huaman, C., et al.
Infections with rabies virus (RABV) and related lyssaviruses are uniformly fatal once virus accesses the central nervous system (CNS) and causes disease signs. Current immunotherapies are thus focused on the early, pre-symptomatic stage of disease, with the goal of peripheral neutralization of virus to prevent CNS infection. Here, we evaluated the therapeutic efficacy of F11, an anti-lyssavirus human monoclonal antibody (mAb), on established lyssavirus infections. We show that a single dose of F11 limits viral load in the brain and reverses disease signs following infection with a lethal dose of lyssavirus, even when administered after initiation of robust virus replication in the CNS. Importantly, we found that F11-dependent neutralization is not sufficient to protect animals from mortality, and a CD4 T cell-dependent adaptive immune response is required for successful control of infection. F11 significantly changes the spectrum of leukocyte populations in the brain, and the FcRγ-binding function of F11 contributes to therapeutic efficacy. Thus, mAb therapy can drive potent neutralization-independent T cell-mediated effects, even against an established CNS infection by a lethal neurotropic virus. © 2023 Commonwealth of Australia and The Authors. Published under the terms of the CC BY 4.0 license. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
MFSD2A potentiates gastric cancer response to anti-PD-1 immunotherapy by reprogramming the tumor microenvironment to activate T cell response.
In Cancer Communications (London, England) on 1 October 2023 by Zhang, B., Wang, C. M., et al.
The efficacy of anti-programmed cell death protein 1 (PD-1) immunotherapy in various cancers, including gastric cancer (GC), needs to be potentiated by more effective targeting to enhance therapeutic efficacy or identifying accurate biomarkers to predict clinical responses. Here, we attempted to identify molecules predicting or/and promoting anti-PD-1 therapeutic response in advanced GC (AGC). The transcriptome of AGC tissues from patients with different clinical responses to anti-PD-1 immunotherapy and GC cells was analyzed by RNA sequencing. The protein and mRNA levels of the major facilitator superfamily domain containing 2A (MFSD2A) in GC cells were assessed via quantitative real-time polymerase chain reaction, Western blotting, and immunohistochemistry. Additionally, the regulation of anti-PD-1 response by MFSD2A was studied in tumor-bearing mice. Cytometry by Time-of-Flight, multiple immunohistochemistry, and flow cytometry assays were used to explore immunological responses. The effects of MFSD2A on lipid metabolism in mice cancer tissue and GC cells was detected by metabolomics. Higher expression of MFSD2A in tumor tissues of AGC patients was associated with better response to anti-PD-1 immunotherapy. Moreover, MFSD2A expression was lower in GC tissues compared to adjacent normal tissues, and its expression was inversely correlated with GC stage. The overexpression of MFSD2A in GC cells enhanced the efficacy of anti-PD-1 immunotherapy in vivo by reprogramming the tumor microenvironment (TME), characterized by increased CD8+ T cell activation and reduced its exhaustion. MFSD2A inhibited transforming growth factor β1 (TGFβ1) release from GC cells by suppressing cyclooxygenase 2 (COX2)-prostaglandin synthesis, which consequently reprogrammed TME to promote anti-tumor T cell activation. MFSD2A potentially serves as a predictive biomarker for anti-PD-1 immunotherapy response in AGC patients. MFSD2A may be a promising therapeutic target to potentiate the efficacy of anti-PD-1 immunotherapy by reprogramming the TME to promote T cells activation. © 2023 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
- Mus musculus (House mouse),
- Biochemistry and Molecular biology,
- Immunology and Microbiology
Vaccination with post-translational modified, homocitrullinated peptides induces CD8 T-cell responses that mediate antitumor immunity.
In Journal for Immunotherapy of Cancer on 1 October 2023 by Shah, S., Cook, K. W., et al.
Post-translational modification of proteins has the potential to alter the ability of T cells to recognize major histocompatibility complex (MHC) class -I and class-II restricted antigens, thereby resulting in altered immune responses. One such modification is carbamylation (homocitrullination) that results in the formation of homocitrulline (Hcit) residues in a non-enzymatic reaction of cyanate with the lysine residues in the polypeptide chain. Homocitrullination occurs in the tumor microenvironment and CD4-mediated immune responses to Hcit epitopes can target stressed tumor cells and provide a potent antitumor response in mouse models. Homocitrullinated peptides were identified and assessed in vitro for HLA-A2 binding and in vivo in human leukocyte antigen (HLA) transgenic mouse models for immunogenicity. CD8 responses were assessed in vitro for cytotoxicity and in vivo tumor therapy. Human tumor samples were analyzed by targeted mass spectrometry for presence of homocitrullinated peptides. Homocitrullinated peptides from aldolase and cytokeratin were identified, that stimulated CD8-mediated responses in vivo. Modified peptides showed enhanced binding to HLA-A2 compared with the native sequences and immunization of HLA-A2 transgenic mice generated high avidity modification specific CD8 responses that killed peptide expressing target cells. Importantly, in vivo the homocitrullinated aldolase specific response was associated with efficient CD8 dependent antitumor therapy of the aggressive murine B16 tumor model indicating that this epitope is naturally presented in the tumor. In addition, the homocitrullinated aldolase epitope was also detected in human tumor samples. This is the first evidence that homocitrullinated peptides can be processed and presented via MHC-I and targeted for tumor therapy. Thus, Hcit-specific CD8 T-cell responses have potential in the development of future anticancer therapy. © Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.