Functional Antibodies: Principles for Translational Confidence
Introduction
First described in 1890, antibodies, also known as immunoglobulins (Ig), are Y-shaped glycoproteins produced by the immune system to recognize and neutralize foreign antigens such as bacteria, viruses, and toxins. Their remarkable specificity and affinity make them fundamental to immune protection and indispensable tools in biomedical research, diagnostics, and therapeutic development.
Antibody Structure
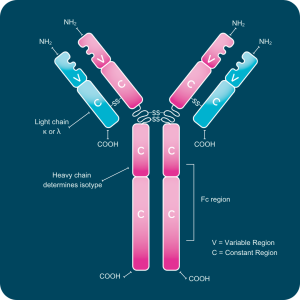 Each antibody molecule consists of two identical heavy chains and two identical light chains. Light chains are categorized as kappa (κ) or lambda (λ) based on sequence differences, while the heavy chain defines the antibody’s isotype.
Each antibody molecule consists of two identical heavy chains and two identical light chains. Light chains are categorized as kappa (κ) or lambda (λ) based on sequence differences, while the heavy chain defines the antibody’s isotype.
Every chain contains one variable (V) domain and one or more constant (C) domains, each composed of roughly 110–130 amino acids. Light chains have a single constant domain, and heavy chains contain three or four. Heavy chains with three constant domains include a flexible hinge region that allows movement of the antigen-binding arms.
A typical light chain has a mass of about 25 kDa, and a heavy chain with three constant domains and a hinge region has a mass near 55 kDa.
Antibody Isotypes
Antibody isotypes are defined by differences in the heavy-chain constant region, which influence disulfide bonding, glycosylation, and the number of constant domains.
In mammals, five major isotypes exist: IgG, IgM, IgA, IgD, and IgE. In humans and mice, IgG is further divided into subclasses (for example, mouse IgG1, IgG2a, IgG2b, IgG2c, and IgG3). Each isotype contributes distinct biological functions, effector mechanisms, and tissue distributions.
| IgG | IgD | IgE | IgA | IgM |
|---|---|---|---|---|
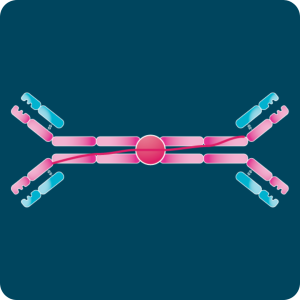
IgG is the most abundant isotype in serum and exhibits the longest half-life of all immunoglobulins. It includes four subclasses with structural and functional distinctions. IgG1 and IgG3 typically target protein antigens, while IgG2 and IgG4 respond to polysaccharide antigens. Because of its stability and versatility, IgG is the predominant isotype used in research and therapeutic development. | IgD occurs at very low concentrations and has a short half-life. Its exact function remains under investigation, but evidence suggests a role in B-cell activation and regulation. | IgE is the least abundant isotype and has the shortest half-life. It mediates allergic hypersensitivity and provides defense against parasitic infections. Despite its low serum levels, IgE’s ability to engage Fc receptors makes it a potent activator of immune responses. | IgA dominates mucosal immunity. It circulates primarily as a monomer but can form dimers joined by a J-chain and a secretory component that protect it from enzymatic degradation. IgA neutralizes pathogens and toxins and prevents their adherence to epithelial surfaces. | IgM is the first antibody produced during a primary immune response. Individual IgM units assemble into pentamers through disulfide bonds and incorporate a J-chain. Although monomeric IgM has low affinity, the pentameric form achieves high avidity by binding multiple epitopes simultaneously. |
 |  |  |  |  |
F(ab) and Fc Fragments
Each antibody contains two F(ab) regions and one Fc region. The F(ab) regions form the antigen-binding arms, while the Fc region mediates effector functions through receptor and complement interactions.
Papain digestion yields two F(ab) fragments and one Fc fragment. Pepsin digestion produces an intact F(ab′)₂ fragment and a degraded Fc fragment. Because F(ab) and F(ab′)₂ fragments retain antigen-binding capability without Fc-mediated activity, they are useful in imaging, blocking assays, and detection applications. 
Conclusion
Antibodies are central to both immune defense and experimental biology. Their structural diversity and functional specialization enable precise investigation of signaling, modulation, and therapeutic mechanisms across in vivo, ex vivo, and in vitro systems. These properties make antibodies essential tools for advancing discovery in immunology, oncology, and translational research.
Meet Our Leaders
Learn more about the team behind the gold standard in vivo antibodies.
Discover Bio X Cell
Learn more about our proven expertise and comprehensive antibody solutions.
See Our Impact
Discover the Bio X Cell Fund’s mission to improve the health of our community
Explore Our Latest
See our latest articles and whitepapers for scientific insights and ideas
Consult With Bio X Cell to Enable Your Next Breakthrough Discovery
Whether you need antibody customization or high-volume production, Bio X Cell is committed to advancing your therapeutic innovations.
Contact Us
