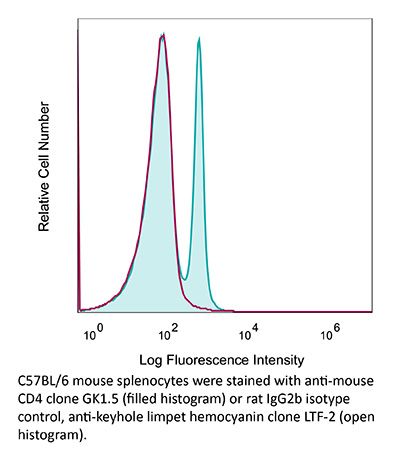
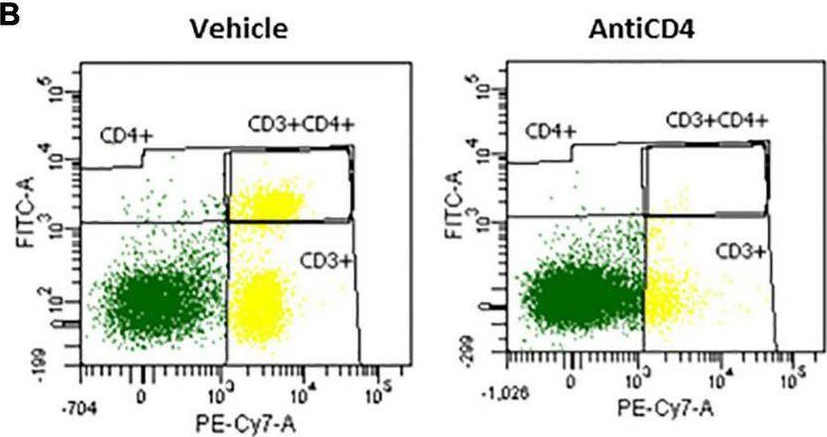
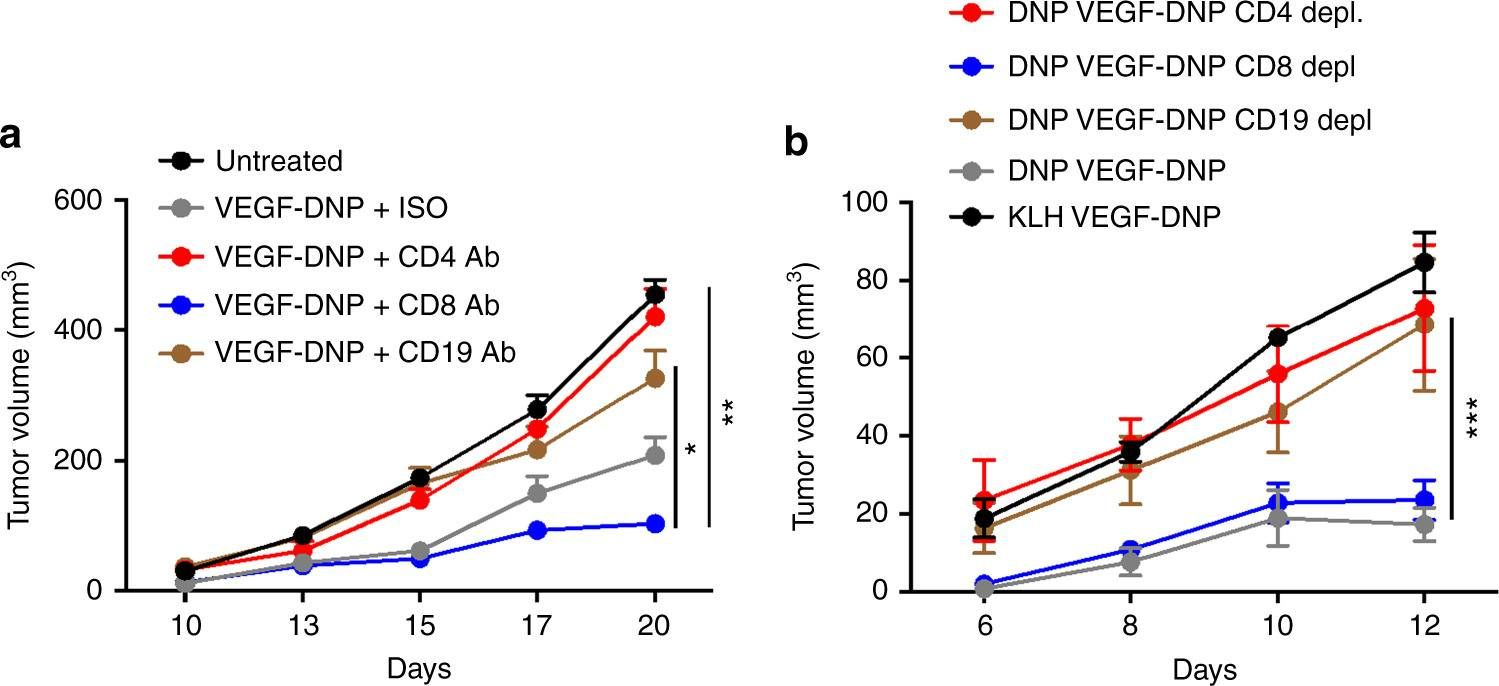
InVivoMAb anti-mouse CD4
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 6.5 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CTL clone V4 |
| Reported Applications |
in vivo CD4+ T cell depletion Flow cytometry Western blot |
| Formulation |
PBS, pH 6.5 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107636 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo NK cell depletion
in vivo CD4+ T cell depletion
in vivo CD8+ T cell depletion
in vivo CTLA-4 neutralization
in vivo NKG2D blockade
Hervieu, A., et al (2013). "Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth" J Invest Dermatol 133(2): 499-508.
PubMed
Dacarbazine (DTIC) is a cytotoxic drug widely used for melanoma treatment. However, the putative contribution of anticancer immune responses in the efficacy of DTIC has not been evaluated. By testing how DTIC affects host immune responses to cancer in a mouse model of melanoma, we unexpectedly found that both natural killer (NK) and CD8(+) T cells were indispensable for DTIC therapeutic effect. Although DTIC did not directly affect immune cells, it triggered the upregulation of NKG2D ligands on tumor cells, leading to NK cell activation and IFNgamma secretion in mice and humans. NK cell-derived IFNgamma subsequently favored upregulation of major histocompatibility complex class I molecules on tumor cells, rendering them sensitive to cytotoxic CD8(+) T cells. Accordingly, DTIC markedly enhanced cytotoxic T lymphocyte antigen 4 inhibition efficacy in vivo in an NK-dependent manner. These results underscore the immunogenic properties of DTIC and provide a rationale to combine DTIC with immunotherapeutic agents that relieve immunosuppression in vivo.
in vivo NK cell depletion
in vivo CD4+ T cell depletion
in vivo CD8+ T cell depletion
in vivo CTLA-4 neutralization
in vivo NKG2D blockade
Hervieu, A., et al (2013). "Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth" J Invest Dermatol 133(2): 499-508.
PubMed
Dacarbazine (DTIC) is a cytotoxic drug widely used for melanoma treatment. However, the putative contribution of anticancer immune responses in the efficacy of DTIC has not been evaluated. By testing how DTIC affects host immune responses to cancer in a mouse model of melanoma, we unexpectedly found that both natural killer (NK) and CD8(+) T cells were indispensable for DTIC therapeutic effect. Although DTIC did not directly affect immune cells, it triggered the upregulation of NKG2D ligands on tumor cells, leading to NK cell activation and IFNgamma secretion in mice and humans. NK cell-derived IFNgamma subsequently favored upregulation of major histocompatibility complex class I molecules on tumor cells, rendering them sensitive to cytotoxic CD8(+) T cells. Accordingly, DTIC markedly enhanced cytotoxic T lymphocyte antigen 4 inhibition efficacy in vivo in an NK-dependent manner. These results underscore the immunogenic properties of DTIC and provide a rationale to combine DTIC with immunotherapeutic agents that relieve immunosuppression in vivo.
in vivo activation of 4-1BB
in vivo CD8+ T cell depletion
in vivo NK cell depletion
in vivo CD4+ T cell depletion
Flow Cytometry
in vivo CTLA-4 neutralization
in vivo CD19 neutralization
Flow Cytometry
Dai, M., et al (2013). "Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation" J Immunother 36(4): 248-257.
PubMed
40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Mice with intraperitoneal ID8 ovarian carcinoma or subcutaneous SW1 melanoma were injected with monoclonal antibodies (mAbs) to CD137PD-1CTLA4 7-15 days after tumor initiation. Survival of mice with ID8 tumors tripled and >40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.
in vivo activation of 4-1BB
in vivo CD8+ T cell depletion
in vivo NK cell depletion
in vivo CD4+ T cell depletion
Flow Cytometry
in vivo CTLA-4 neutralization
in vivo CD19 neutralization
Flow Cytometry
Dai, M., et al (2013). "Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation" J Immunother 36(4): 248-257.
PubMed
40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Mice with intraperitoneal ID8 ovarian carcinoma or subcutaneous SW1 melanoma were injected with monoclonal antibodies (mAbs) to CD137PD-1CTLA4 7-15 days after tumor initiation. Survival of mice with ID8 tumors tripled and >40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.
in vivo macrophage depletion
in vivo blocking of PD-1/PD-L signaling
in vivo NK cell depletion
in vivo CD4+ T cell depletion
in vivo CD8+ T cell depletion
in vivo neutrophil depletion
in vivo eosinophil depletion
Moynihan, K. D., et al (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200.
PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo macrophage depletion
in vivo blocking of PD-1/PD-L signaling
in vivo NK cell depletion
in vivo CD4+ T cell depletion
in vivo CD8+ T cell depletion
in vivo neutrophil depletion
in vivo eosinophil depletion
Moynihan, K. D., et al (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200.
PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo IL-17A neutralization
in vivo IFNγ neutralization
Flow Cytometry
in vivo NK cell depletion
Flow Cytometry
in vivo CD4+ T cell depletion
Flow Cytometry
in vivo CD8+ T cell depletion
Flow Cytometry
Uddin, M. N., et al (2014). "TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma" J Immunol 192(4): 1946-1953.
PubMed
Using several tumor models, we demonstrate that mice deficient in Bcl11b in T cells, although having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared with wild-type mice. Bcl11b(-/-) CD4(+) T cells, with elevated TNF-alpha levels, but not the Bcl11b(-/-) CD8(+) T cells, were required for the reduced tumor burden, as were NK1.1(+) cells, found in increased numbers in Bcl11b(F/F)/CD4-Cre mice. Among NK1.1(+) cells, the NK cell population was predominant in number and was the only population displaying elevated granzyme B levels and increased degranulation, although not increased proliferation. Although the number of myeloid-derived suppressor cells was increased in the lungs with metastatic tumors of Bcl11b(F/F)/CD4-Cre mice, their arginase-1 levels were severely reduced. The increase in NK cell and myeloid-derived suppressor cell numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung, and increased hematopoiesis in Bcl11b(F/F)/CD4-Cre mice were all dependent on TNF-alpha. Moreover, TNF-alpha treatment of wild-type mice also reduced the tumor burden and increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNF-alpha of lineage-negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNF-alpha in promoting hematopoiesis. These studies reveal a novel role for TNF-alpha in the antitumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
in vivo blocking of PD-1/PD-L signaling
in vivo CD4+ T cell depletion
in vivo CD8+ T cell depletion
Vanpouille-Box, C., et al (2015). "TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity" Cancer Res 75(11): 2232-2242.
PubMed
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient’s individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFbeta activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFbeta neutralization during radiation therapy effectively generates CD8(+) T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNgamma and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFbeta blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFbeta blockade. Thus, TGFbeta is a fundamental regulator of radiation therapy’s ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFbeta neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
in vivo blocking of PD-1/PD-L signaling
in vivo CD8+ T cell depletion
Evans, E. E., et al (2015). "Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies" Cancer Immunol Res 3(6): 689-701.
PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
in vivo TNFα neutralization
in vivo regulatory T cell depletion
in vivo CD4+ T cell depletion
in vivo CD8+ T cell depletion
in vivo blocking of IL-10/IL-10R signaling
Christensen, A. D., et al (2015). "Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells" Clin Exp Immunol 179(3): 485-499.
PubMed
Regulatory T cells (Tregs ) are known to play an immunosuppressive role in the response of contact hypersensitivity (CHS), but neither the dynamics of Tregs during the CHS response nor the exaggerated inflammatory response after depletion of Tregs has been characterized in detail. In this study we show that the number of Tregs in the challenged tissue peak at the same time as the ear-swelling reaches its maximum on day 1 after challenge, whereas the number of Tregs in the draining lymph nodes peaks at day 2. As expected, depletion of Tregs by injection of a monoclonal antibody to CD25 prior to sensitization led to a prolonged and sustained inflammatory response which was dependent upon CD8 T cells, and co-stimulatory blockade with cytotoxic T lymphocyte antigen-4-immunoglobulin (CTLA-4-Ig) suppressed the exaggerated inflammation. In contrast, blockade of the interleukin (IL)-10-receptor (IL-10R) did not further increase the exaggerated inflammatory response in the Treg -depleted mice. In the absence of Tregs , the response changed from a mainly acute reaction with heavy infiltration of neutrophils to a sustained response with more chronic characteristics (fewer neutrophils and dominated by macrophages). Furthermore, depletion of Tregs enhanced the release of cytokines and chemokines locally in the inflamed ear and augmented serum levels of the systemic inflammatory mediators serum amyloid (SAP) and haptoglobin early in the response.
in vivo CD8+ T cell depletion
in vivo CD4+ T cell depletion
Flow Cytometry
Krieg, C., et al (2010). "Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells" Proc Natl Acad Sci U S A 107(26): 11906-11911.
PubMed
IL-2 immunotherapy is an attractive treatment option for certain metastatic cancers. However, administration of IL-2 to patients can lead, by ill-defined mechanisms, to toxic adverse effects including severe pulmonary edema. Here, we show that IL-2-induced pulmonary edema is caused by direct interaction of IL-2 with functional IL-2 receptors (IL-2R) on lung endothelial cells in vivo. Treatment of mice with high-dose IL-2 led to efficient expansion of effector immune cells expressing high levels of IL-2Rbetagamma, including CD8(+) T cells and natural killer cells, which resulted in a considerable antitumor response against s.c. and pulmonary B16 melanoma nodules. However, high-dose IL-2 treatment also affected immune cell lineage marker-negative CD31(+) pulmonary endothelial cells via binding to functional alphabetagamma IL-2Rs, expressed at low to intermediate levels on these cells, thus causing pulmonary edema. Notably, IL-2-mediated pulmonary edema was abrogated by a blocking antibody to IL-2Ralpha (CD25), genetic disruption of CD25, or the use of IL-2Rbetagamma-directed IL-2/anti-IL-2 antibody complexes, thereby interfering with IL-2 binding to IL-2Ralphabetagamma(+) pulmonary endothelial cells. Moreover, IL-2/anti-IL-2 antibody complexes led to vigorous activation of IL-2Rbetagamma(+) effector immune cells, which generated a dramatic antitumor response. Thus, IL-2/anti-IL-2 antibody complexes might improve current strategies of IL-2-based tumor immunotherapy.
in vivo IL-17A neutralization
in vivo CD4+ T cell depletion
in vivo ICOSL neutralization
in vivo blocking of OX40/OX40L signaling
Xin, L., et al (2014). "Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2" Proc Natl Acad Sci U S A 111(29): 10672-10677.
PubMed
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4(+) T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4(+) T cells with commensal microbe specificity.
in vivo IL-17A neutralization
in vivo IFNγ neutralization
Flow Cytometry
in vivo NK cell depletion
Flow Cytometry
in vivo CD4+ T cell depletion
Flow Cytometry
in vivo CD8+ T cell depletion
Flow Cytometry
Uddin, M. N., et al (2014). "TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma" J Immunol 192(4): 1946-1953.
PubMed
Using several tumor models, we demonstrate that mice deficient in Bcl11b in T cells, although having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared with wild-type mice. Bcl11b(-/-) CD4(+) T cells, with elevated TNF-alpha levels, but not the Bcl11b(-/-) CD8(+) T cells, were required for the reduced tumor burden, as were NK1.1(+) cells, found in increased numbers in Bcl11b(F/F)/CD4-Cre mice. Among NK1.1(+) cells, the NK cell population was predominant in number and was the only population displaying elevated granzyme B levels and increased degranulation, although not increased proliferation. Although the number of myeloid-derived suppressor cells was increased in the lungs with metastatic tumors of Bcl11b(F/F)/CD4-Cre mice, their arginase-1 levels were severely reduced. The increase in NK cell and myeloid-derived suppressor cell numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung, and increased hematopoiesis in Bcl11b(F/F)/CD4-Cre mice were all dependent on TNF-alpha. Moreover, TNF-alpha treatment of wild-type mice also reduced the tumor burden and increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNF-alpha of lineage-negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNF-alpha in promoting hematopoiesis. These studies reveal a novel role for TNF-alpha in the antitumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
in vivo blocking of PD-1/PD-L signaling
in vivo CD4+ T cell depletion
in vivo CD8+ T cell depletion
Vanpouille-Box, C., et al (2015). "TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity" Cancer Res 75(11): 2232-2242.
PubMed
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient’s individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFbeta activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFbeta neutralization during radiation therapy effectively generates CD8(+) T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNgamma and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFbeta blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFbeta blockade. Thus, TGFbeta is a fundamental regulator of radiation therapy’s ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFbeta neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
in vivo IL-17A neutralization
in vivo CD4+ T cell depletion
in vivo ICOSL neutralization
in vivo blocking of OX40/OX40L signaling
Xin, L., et al (2014). "Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2" Proc Natl Acad Sci U S A 111(29): 10672-10677.
PubMed
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4(+) T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4(+) T cells with commensal microbe specificity.
in vivo blocking of PD-1/PD-L signaling
in vivo CD8+ T cell depletion
Evans, E. E., et al (2015). "Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies" Cancer Immunol Res 3(6): 689-701.
PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
in vivo TNFα neutralization
in vivo regulatory T cell depletion
in vivo CD4+ T cell depletion
in vivo CD8+ T cell depletion
in vivo blocking of IL-10/IL-10R signaling
Christensen, A. D., et al (2015). "Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells" Clin Exp Immunol 179(3): 485-499.
PubMed
Regulatory T cells (Tregs ) are known to play an immunosuppressive role in the response of contact hypersensitivity (CHS), but neither the dynamics of Tregs during the CHS response nor the exaggerated inflammatory response after depletion of Tregs has been characterized in detail. In this study we show that the number of Tregs in the challenged tissue peak at the same time as the ear-swelling reaches its maximum on day 1 after challenge, whereas the number of Tregs in the draining lymph nodes peaks at day 2. As expected, depletion of Tregs by injection of a monoclonal antibody to CD25 prior to sensitization led to a prolonged and sustained inflammatory response which was dependent upon CD8 T cells, and co-stimulatory blockade with cytotoxic T lymphocyte antigen-4-immunoglobulin (CTLA-4-Ig) suppressed the exaggerated inflammation. In contrast, blockade of the interleukin (IL)-10-receptor (IL-10R) did not further increase the exaggerated inflammatory response in the Treg -depleted mice. In the absence of Tregs , the response changed from a mainly acute reaction with heavy infiltration of neutrophils to a sustained response with more chronic characteristics (fewer neutrophils and dominated by macrophages). Furthermore, depletion of Tregs enhanced the release of cytokines and chemokines locally in the inflamed ear and augmented serum levels of the systemic inflammatory mediators serum amyloid (SAP) and haptoglobin early in the response.
in vivo CD8+ T cell depletion
in vivo CD4+ T cell depletion
Flow Cytometry
Krieg, C., et al (2010). "Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells" Proc Natl Acad Sci U S A 107(26): 11906-11911.
PubMed
IL-2 immunotherapy is an attractive treatment option for certain metastatic cancers. However, administration of IL-2 to patients can lead, by ill-defined mechanisms, to toxic adverse effects including severe pulmonary edema. Here, we show that IL-2-induced pulmonary edema is caused by direct interaction of IL-2 with functional IL-2 receptors (IL-2R) on lung endothelial cells in vivo. Treatment of mice with high-dose IL-2 led to efficient expansion of effector immune cells expressing high levels of IL-2Rbetagamma, including CD8(+) T cells and natural killer cells, which resulted in a considerable antitumor response against s.c. and pulmonary B16 melanoma nodules. However, high-dose IL-2 treatment also affected immune cell lineage marker-negative CD31(+) pulmonary endothelial cells via binding to functional alphabetagamma IL-2Rs, expressed at low to intermediate levels on these cells, thus causing pulmonary edema. Notably, IL-2-mediated pulmonary edema was abrogated by a blocking antibody to IL-2Ralpha (CD25), genetic disruption of CD25, or the use of IL-2Rbetagamma-directed IL-2/anti-IL-2 antibody complexes, thereby interfering with IL-2 binding to IL-2Ralphabetagamma(+) pulmonary endothelial cells. Moreover, IL-2/anti-IL-2 antibody complexes led to vigorous activation of IL-2Rbetagamma(+) effector immune cells, which generated a dramatic antitumor response. Thus, IL-2/anti-IL-2 antibody complexes might improve current strategies of IL-2-based tumor immunotherapy.
in vivo CD4+ T cell depletion
in vivo PD-L1 blockade
in vivo T cell depletion
Kim, J., et al (2015). "Memory programming in CD8(+) T-cell differentiation is intrinsic and is not determined by CD4 help" Nat Commun 6: 7994.
PubMed
CD8(+) T cells activated without CD4(+) T-cell help are impaired in memory expansion. To understand the underlying cellular mechanism, here we track the dynamics of helper-deficient CD8(+) T-cell response to a minor histocompatibility antigen by phenotypic and in vivo imaging analyses. Helper-deficient CD8(+) T cells show reduced burst expansion, rapid peripheral egress, delayed antigen clearance and continuous activation, and are eventually exhausted. Contrary to the general consensus that CD4 help encodes memory programmes in CD8(+) T cells and helper-deficient CD8(+) T cells are abortive, these cells can differentiate into effectors and memory precursors. Importantly, accelerating antigen clearance or simply increasing the burst effector size enables generation of memory cells by CD8(+) T cells, regardless of CD4 help. These results suggest that the memory programme is CD8(+) T-cell-intrinsic, and provide insight into the role of CD4 help in CD8(+) T-cell responses.
in vivo CD4+ T cell depletion
Butler, N. S., et al (2012). "Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection" Nat Immunol 13(2): 188-195.
PubMed
Infection of erythrocytes with Plasmodium species induces clinical malaria. Parasite-specific CD4(+) T cells correlate with lower parasite burdens and severity of human malaria and are needed to control blood-stage infection in mice. However, the characteristics of CD4(+) T cells that determine protection or parasite persistence remain unknown. Here we show that infection of humans with Plasmodium falciparum resulted in higher expression of the inhibitory receptor PD-1 associated with T cell dysfunction. In vivo blockade of the PD-1 ligand PD-L1 and the inhibitory receptor LAG-3 restored CD4(+) T cell function, amplified the number of follicular helper T cells and germinal-center B cells and plasmablasts, enhanced protective antibodies and rapidly cleared blood-stage malaria in mice. Thus, chronic malaria drives specific T cell dysfunction, and proper function can be restored by inhibitory therapies to enhance parasite control.
in vivo CD4+ T cell depletion
Church, S. E., et al (2014). "Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells" Eur J Immunol 44(1): 69-79.
PubMed
Immunotherapies that augment antitumor T cells have had recent success for treating patients with cancer. Here we examined whether tumor-specific CD4(+) T cells enhance CD8(+) T-cell adoptive immunotherapy in a lymphopenic environment. Our model employed physiological doses of tyrosinase-related protein 1-specific CD4(+) transgenic T cells-CD4(+) T cells and pmel-CD8(+) T cells that when transferred individually were subtherapeutic; however, when transferred together provided significant (p
in vivo CD4+ T cell depletion
Flow Cytometry
in vivo blocking of IL-10/IL-10R signaling
Liu, G., et al (2015). "IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4+ T Cells and IFN-gamma" PLoS Pathog 11(7): e1005065.
PubMed
African trypanosomes are extracellular protozoan parasites causing a chronic debilitating disease associated with a persistent inflammatory response. Maintaining the balance of the inflammatory response via downregulation of activation of M1-type myeloid cells was previously shown to be crucial to allow prolonged survival. Here we demonstrate that infection with African trypanosomes of IL-27 receptor-deficient (IL-27R-/-) mice results in severe liver immunopathology and dramatically reduced survival as compared to wild-type mice. This coincides with the development of an exacerbated Th1-mediated immune response with overactivation of CD4+ T cells and strongly enhanced production of inflammatory cytokines including IFN-gamma. What is important is that IL-10 production was not impaired in infected IL-27R-/- mice. Depletion of CD4+ T cells in infected IL-27R-/- mice resulted in a dramatically reduced production of IFN-gamma, preventing the early mortality of infected IL-27R-/- mice. This was accompanied by a significantly reduced inflammatory response and a major amelioration of liver pathology. These results could be mimicked by treating IL-27R-/- mice with a neutralizing anti-IFN-gamma antibody. Thus, our data identify IL-27 signaling as a novel pathway to prevent early mortality via inhibiting hyperactivation of CD4+ Th1 cells and their excessive secretion of IFN-gamma during infection with African trypanosomes. These data are the first to demonstrate the essential role of IL-27 signaling in regulating immune responses to extracellular protozoan infections.
in vivo CD4+ T cell depletion
in vivo PD-L1 blockade
in vivo T cell depletion
Kim, J., et al (2015). "Memory programming in CD8(+) T-cell differentiation is intrinsic and is not determined by CD4 help" Nat Commun 6: 7994.
PubMed
CD8(+) T cells activated without CD4(+) T-cell help are impaired in memory expansion. To understand the underlying cellular mechanism, here we track the dynamics of helper-deficient CD8(+) T-cell response to a minor histocompatibility antigen by phenotypic and in vivo imaging analyses. Helper-deficient CD8(+) T cells show reduced burst expansion, rapid peripheral egress, delayed antigen clearance and continuous activation, and are eventually exhausted. Contrary to the general consensus that CD4 help encodes memory programmes in CD8(+) T cells and helper-deficient CD8(+) T cells are abortive, these cells can differentiate into effectors and memory precursors. Importantly, accelerating antigen clearance or simply increasing the burst effector size enables generation of memory cells by CD8(+) T cells, regardless of CD4 help. These results suggest that the memory programme is CD8(+) T-cell-intrinsic, and provide insight into the role of CD4 help in CD8(+) T-cell responses.
in vivo CD4+ T cell depletion
in vivo blockade of TCR stimulation
Guo, L., et al (2015). "Innate immunological function of TH2 cells in vivo" Nat Immunol 16(10): 1051-1059.
PubMed
Type 2 helper T cells (TH2 cells) produce interleukin 13 (IL-13) when stimulated by papain or house dust mite extract (HDM) and induce eosinophilic inflammation. This innate response is dependent on IL-33 but not T cell antigen receptors (TCRs). While type 2 innate lymphoid cells (ILC2 cells) are the dominant innate producers of IL-13 in naive mice, we found here that helminth-infected mice had more TH2 cells compared to uninfected mice, and thes e cells became major mediators of innate type 2 responses. TH2 cells made important contributions to HDM-induced antigen-nonspecific eosinophilic inflammation and protected mice recovering from infection with Ascaris suum against subsequent infection with the phylogenetically distant nematode Nippostrongylus brasiliensis. Our findings reveal a previously unappreciated role for effector TH2 cells during TCR-independent innate-like immune responses.
in vivo CD4+ T cell depletion
Budda, S. A. and L. A. Zenewicz (2018). "IL-22 deficiency increases CD4 T cell responses to mucosal immunization" Vaccine 36(25): 3694-3700.
PubMed
Mucosal vaccines are a promising platform for combatting infectious diseases for which we still lack effective preventative measures. Optimizing these vaccines to generate the best protective immune responses with the least complicated immunization regimen is imperative. Mucosal barriers are the first line of defense against many pathogens and, as such, we looked to their biology for strategies to improve vaccine delivery. Interleukin-22 (IL-22) is a key cytokine in both healthy and inflamed mucosal tissues. IL-22 promotes epithelial cell proliferation and inhibits apoptosis, upregulates mucin and antimicrobial peptides, all of which promote mucosal barrier integrity. In this study, we find that IL-22 impairs the development of a T cell response during mucosal immunization. Compared to wild-type control mice, IL-22 deficient mice had increased antigen-specific CD4 T cell responses to intrarectal immunization using a protein and cholera toxin adjuvant vaccine. When immunized systemically with the same protein antigen adsorbed to alum, no differences in the CD4 T cell response between wild-type and IL-22 deficient mice were detected. This suggests that transiently inhibiting IL-22 during mucosal vaccination could enhance T cell responses. The broad-applicability of this proposed approach would allow for improvement of many existing mucosal vaccine regimens and have positive implications in the development of more efficacious mucosal vaccines.
in vivo CD4+ T cell depletion
Flow Cytometry
in vivo CD8+ T cell depletion
Balogh, K. N., et al (2018). "Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses" PLoS One 13(6): e0197702.
PubMed
The Macrophage Migration Inhibitory Factor (MIF) is an inflammatory cytokine that is overexpressed in a number of cancer types, with increased MIF expression often correlating with tumor aggressiveness and poor patient outcomes. In this study, we aimed to better understand the link between primary tumor expression of MIF and increased tumor growth. Using the MMTV-PyMT murine model of breast cancer, we observed that elevated MIF expression promoted tumor appearance and growth. Supporting this, we confirmed our previous observation that higher MIF expression supported tumor growth in the 4T1 murine model of breast cancer. We subsequently discovered that loss of MIF expression in 4T1 cells led to decreased cell numbers and increased apoptosis in vitro under reduced serum culture conditions. We hypothesized that this increase in cell death would promote detection by the host immune system in vivo, which could explain the observed impairment in tumor growth. Supporting this, we demonstrated that loss of MIF expression in the primary tumor led to an increased abundance of intra-tumoral IFNgamma-producing CD4+ and CD8+ T cells, and that depletion of T cells from mice bearing MIF-deficient tumors restored growth to the level of MIF-expressing tumors. Furthermore, we found that MIF depletion from the tumor cells resulted in greater numbers of activated intra-tumoral dendritic cells (DCs). Lastly, we demonstrated that loss of MIF expression led to a robust induction of a specialized form of cell death, immunogenic cell death (ICD), in vitro. Together, our data suggests a model in which MIF expression in the primary tumor dampens the anti-tumor immune response, promoting tumor growth.
in vivo OX40 activation
in vivo IFNγ neutralization
in vivo blocking of PD-1/PD-L signaling
in vivo CD4+ T cell depletion
in vivo PD-L1 blockade
Zander, R. A., et al (2015). "PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity" Cell Host Microbe 17(5): 628-641.
PubMed
The differentiation and protective capacity of Plasmodium-specific T cells are regulated by both positive and negative signals during malaria, but the molecular and cellular details remain poorly defined. Here we show that malaria patients and Plasmodium-infected rodents exhibit atypical expression of the co-stimulatory receptor OX40 on CD4 T cells and that therapeutic enhancement of OX40 signaling enhances helper CD4 T cell activity, humoral immunity, and parasite clearance in rodents. However, these beneficial effects of OX40 signaling are abrogated following coordinate blockade of PD-1 co-inhibitory pathways, which are also upregulated during malaria and associated with elevated parasitemia. Co-administration of biologics blocking PD-1 and promoting OX40 signaling induces excessive interferon-gamma that directly limits helper T cell-mediated support of humoral immunity and decreases parasite control. Our results show that targeting OX40 can enhance Plasmodium control and that crosstalk between co-inhibitory and co-stimulatory pathways in pathogen-specific CD4 T cells can impact pathogen clearance.
in vivo OX40 activation
in vivo CD8+ T cell depletion
in vivo CD4+ T cell depletion
in vivo blocking of ICOS/ICOSL signaling
In vivo CD70 blockade
Krupnick, A. S., et al (2014). "Central memory CD8+ T lymphocytes mediate lung allograft acceptance" J Clin Invest 124(3): 1130-1143.
PubMed
Memory T lymphocytes are commonly viewed as a major barrier for long-term survival of organ allografts and are thought to accelerate rejection responses due to their rapid infiltration into allografts, low threshold for activation, and ability to produce inflammatory mediators. Because memory T cells are usually associated with rejection, preclinical protocols have been developed to target this population in transplant recipients. Here, using a murine model, we found that costimulatory blockade-mediated lung allograft acceptance depended on the rapid infiltration of the graft by central memory CD8+ T cells (CD44(hi)CD62L(hi)CCR7+). Chemokine receptor signaling and alloantigen recognition were required for trafficking of these memory T cells to lung allografts. Intravital 2-photon imaging revealed that CCR7 expression on CD8+ T cells was critical for formation of stable synapses with antigen-presenting cells, resulting in IFN-gamma production, which induced NO and downregulated alloimmune responses. Thus, we describe a critical role for CD8+ central memory T cells in lung allograft acceptance and highlight the need for tailored approaches for tolerance induction in the lung.
in vivo OX40 activation
in vivo IFNγ neutralization
in vivo blocking of PD-1/PD-L signaling
in vivo CD4+ T cell depletion
in vivo PD-L1 blockade
Zander, R. A., et al (2015). "PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity" Cell Host Microbe 17(5): 628-641.
PubMed
The differentiation and protective capacity of Plasmodium-specific T cells are regulated by both positive and negative signals during malaria, but the molecular and cellular details remain poorly defined. Here we show that malaria patients and Plasmodium-infected rodents exhibit atypical expression of the co-stimulatory receptor OX40 on CD4 T cells and that therapeutic enhancement of OX40 signaling enhances helper CD4 T cell activity, humoral immunity, and parasite clearance in rodents. However, these beneficial effects of OX40 signaling are abrogated following coordinate blockade of PD-1 co-inhibitory pathways, which are also upregulated during malaria and associated with elevated parasitemia. Co-administration of biologics blocking PD-1 and promoting OX40 signaling induces excessive interferon-gamma that directly limits helper T cell-mediated support of humoral immunity and decreases parasite control. Our results show that targeting OX40 can enhance Plasmodium control and that crosstalk between co-inhibitory and co-stimulatory pathways in pathogen-specific CD4 T cells can impact pathogen clearance.
in vivo CD4+ T cell depletion
Flow Cytometry
in vivo CD8+ T cell depletion
Balogh, K. N., et al (2018). "Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses" PLoS One 13(6): e0197702.
PubMed
The Macrophage Migration Inhibitory Factor (MIF) is an inflammatory cytokine that is overexpressed in a number of cancer types, with increased MIF expression often correlating with tumor aggressiveness and poor patient outcomes. In this study, we aimed to better understand the link between primary tumor expression of MIF and increased tumor growth. Using the MMTV-PyMT murine model of breast cancer, we observed that elevated MIF expression promoted tumor appearance and growth. Supporting this, we confirmed our previous observation that higher MIF expression supported tumor growth in the 4T1 murine model of breast cancer. We subsequently discovered that loss of MIF expression in 4T1 cells led to decreased cell numbers and increased apoptosis in vitro under reduced serum culture conditions. We hypothesized that this increase in cell death would promote detection by the host immune system in vivo, which could explain the observed impairment in tumor growth. Supporting this, we demonstrated that loss of MIF expression in the primary tumor led to an increased abundance of intra-tumoral IFNgamma-producing CD4+ and CD8+ T cells, and that depletion of T cells from mice bearing MIF-deficient tumors restored growth to the level of MIF-expressing tumors. Furthermore, we found that MIF depletion from the tumor cells resulted in greater numbers of activated intra-tumoral dendritic cells (DCs). Lastly, we demonstrated that loss of MIF expression led to a robust induction of a specialized form of cell death, immunogenic cell death (ICD), in vitro. Together, our data suggests a model in which MIF expression in the primary tumor dampens the anti-tumor immune response, promoting tumor growth.
in vivo CD4+ T cell depletion
Budda, S. A. and L. A. Zenewicz (2018). "IL-22 deficiency increases CD4 T cell responses to mucosal immunization" Vaccine 36(25): 3694-3700.
PubMed
Mucosal vaccines are a promising platform for combatting infectious diseases for which we still lack effective preventative measures. Optimizing these vaccines to generate the best protective immune responses with the least complicated immunization regimen is imperative. Mucosal barriers are the first line of defense against many pathogens and, as such, we looked to their biology for strategies to improve vaccine delivery. Interleukin-22 (IL-22) is a key cytokine in both healthy and inflamed mucosal tissues. IL-22 promotes epithelial cell proliferation and inhibits apoptosis, upregulates mucin and antimicrobial peptides, all of which promote mucosal barrier integrity. In this study, we find that IL-22 impairs the development of a T cell response during mucosal immunization. Compared to wild-type control mice, IL-22 deficient mice had increased antigen-specific CD4 T cell responses to intrarectal immunization using a protein and cholera toxin adjuvant vaccine. When immunized systemically with the same protein antigen adsorbed to alum, no differences in the CD4 T cell response between wild-type and IL-22 deficient mice were detected. This suggests that transiently inhibiting IL-22 during mucosal vaccination could enhance T cell responses. The broad-applicability of this proposed approach would allow for improvement of many existing mucosal vaccine regimens and have positive implications in the development of more efficacious mucosal vaccines.
in vivo CD4+ T cell depletion
Butler, N. S., et al (2012). "Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection" Nat Immunol 13(2): 188-195.
PubMed
Infection of erythrocytes with Plasmodium species induces clinical malaria. Parasite-specific CD4(+) T cells correlate with lower parasite burdens and severity of human malaria and are needed to control blood-stage infection in mice. However, the characteristics of CD4(+) T cells that determine protection or parasite persistence remain unknown. Here we show that infection of humans with Plasmodium falciparum resulted in higher expression of the inhibitory receptor PD-1 associated with T cell dysfunction. In vivo blockade of the PD-1 ligand PD-L1 and the inhibitory receptor LAG-3 restored CD4(+) T cell function, amplified the number of follicular helper T cells and germinal-center B cells and plasmablasts, enhanced protective antibodies and rapidly cleared blood-stage malaria in mice. Thus, chronic malaria drives specific T cell dysfunction, and proper function can be restored by inhibitory therapies to enhance parasite control.
in vivo CD4+ T cell depletion
Church, S. E., et al (2014). "Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells" Eur J Immunol 44(1): 69-79.
PubMed
Immunotherapies that augment antitumor T cells have had recent success for treating patients with cancer. Here we examined whether tumor-specific CD4(+) T cells enhance CD8(+) T-cell adoptive immunotherapy in a lymphopenic environment. Our model employed physiological doses of tyrosinase-related protein 1-specific CD4(+) transgenic T cells-CD4(+) T cells and pmel-CD8(+) T cells that when transferred individually were subtherapeutic; however, when transferred together provided significant (p
in vivo CD4+ T cell depletion
Flow Cytometry
in vivo blocking of IL-10/IL-10R signaling
Liu, G., et al (2015). "IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4+ T Cells and IFN-gamma" PLoS Pathog 11(7): e1005065.
PubMed
African trypanosomes are extracellular protozoan parasites causing a chronic debilitating disease associated with a persistent inflammatory response. Maintaining the balance of the inflammatory response via downregulation of activation of M1-type myeloid cells was previously shown to be crucial to allow prolonged survival. Here we demonstrate that infection with African trypanosomes of IL-27 receptor-deficient (IL-27R-/-) mice results in severe liver immunopathology and dramatically reduced survival as compared to wild-type mice. This coincides with the development of an exacerbated Th1-mediated immune response with overactivation of CD4+ T cells and strongly enhanced production of inflammatory cytokines including IFN-gamma. What is important is that IL-10 production was not impaired in infected IL-27R-/- mice. Depletion of CD4+ T cells in infected IL-27R-/- mice resulted in a dramatically reduced production of IFN-gamma, preventing the early mortality of infected IL-27R-/- mice. This was accompanied by a significantly reduced inflammatory response and a major amelioration of liver pathology. These results could be mimicked by treating IL-27R-/- mice with a neutralizing anti-IFN-gamma antibody. Thus, our data identify IL-27 signaling as a novel pathway to prevent early mortality via inhibiting hyperactivation of CD4+ Th1 cells and their excessive secretion of IFN-gamma during infection with African trypanosomes. These data are the first to demonstrate the essential role of IL-27 signaling in regulating immune responses to extracellular protozoan infections.
in vivo CD4+ T cell depletion
in vivo blockade of TCR stimulation
Guo, L., et al (2015). "Innate immunological function of TH2 cells in vivo" Nat Immunol 16(10): 1051-1059.
PubMed
Type 2 helper T cells (TH2 cells) produce interleukin 13 (IL-13) when stimulated by papain or house dust mite extract (HDM) and induce eosinophilic inflammation. This innate response is dependent on IL-33 but not T cell antigen receptors (TCRs). While type 2 innate lymphoid cells (ILC2 cells) are the dominant innate producers of IL-13 in naive mice, we found here that helminth-infected mice had more TH2 cells compared to uninfected mice, and thes e cells became major mediators of innate type 2 responses. TH2 cells made important contributions to HDM-induced antigen-nonspecific eosinophilic inflammation and protected mice recovering from infection with Ascaris suum against subsequent infection with the phylogenetically distant nematode Nippostrongylus brasiliensis. Our findings reveal a previously unappreciated role for effector TH2 cells during TCR-independent innate-like immune responses.
in vivo OX40 activation
in vivo CD8+ T cell depletion
in vivo CD4+ T cell depletion
in vivo blocking of ICOS/ICOSL signaling
In vivo CD70 blockade
Krupnick, A. S., et al (2014). "Central memory CD8+ T lymphocytes mediate lung allograft acceptance" J Clin Invest 124(3): 1130-1143.
PubMed
Memory T lymphocytes are commonly viewed as a major barrier for long-term survival of organ allografts and are thought to accelerate rejection responses due to their rapid infiltration into allografts, low threshold for activation, and ability to produce inflammatory mediators. Because memory T cells are usually associated with rejection, preclinical protocols have been developed to target this population in transplant recipients. Here, using a murine model, we found that costimulatory blockade-mediated lung allograft acceptance depended on the rapid infiltration of the graft by central memory CD8+ T cells (CD44(hi)CD62L(hi)CCR7+). Chemokine receptor signaling and alloantigen recognition were required for trafficking of these memory T cells to lung allografts. Intravital 2-photon imaging revealed that CCR7 expression on CD8+ T cells was critical for formation of stable synapses with antigen-presenting cells, resulting in IFN-gamma production, which induced NO and downregulated alloimmune responses. Thus, we describe a critical role for CD8+ central memory T cells in lung allograft acceptance and highlight the need for tailored approaches for tolerance induction in the lung.
in vivo CD4+ T cell depletion
Flow Cytometry
Balogh, K. N., et al (2018). "Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses" PLoS One 13(6): e0197702.
PubMed
The Macrophage Migration Inhibitory Factor (MIF) is an inflammatory cytokine that is overexpressed in a number of cancer types, with increased MIF expression often correlating with tumor aggressiveness and poor patient outcomes. In this study, we aimed to better understand the link between primary tumor expression of MIF and increased tumor growth. Using the MMTV-PyMT murine model of breast cancer, we observed that elevated MIF expression promoted tumor appearance and growth. Supporting this, we confirmed our previous observation that higher MIF expression supported tumor growth in the 4T1 murine model of breast cancer. We subsequently discovered that loss of MIF expression in 4T1 cells led to decreased cell numbers and increased apoptosis in vitro under reduced serum culture conditions. We hypothesized that this increase in cell death would promote detection by the host immune system in vivo, which could explain the observed impairment in tumor growth. Supporting this, we demonstrated that loss of MIF expression in the primary tumor led to an increased abundance of intra-tumoral IFNgamma-producing CD4+ and CD8+ T cells, and that depletion of T cells from mice bearing MIF-deficient tumors restored growth to the level of MIF-expressing tumors. Furthermore, we found that MIF depletion from the tumor cells resulted in greater numbers of activated intra-tumoral dendritic cells (DCs). Lastly, we demonstrated that loss of MIF expression led to a robust induction of a specialized form of cell death, immunogenic cell death (ICD), in vitro. Together, our data suggests a model in which MIF expression in the primary tumor dampens the anti-tumor immune response, promoting tumor growth.
in vivo CD4+ T cell depletion
Budda, S. A. and L. A. Zenewicz (2018). "IL-22 deficiency increases CD4 T cell responses to mucosal immunization" Vaccine 36(25): 3694-3700.
PubMed
Mucosal vaccines are a promising platform for combatting infectious diseases for which we still lack effective preventative measures. Optimizing these vaccines to generate the best protective immune responses with the least complicated immunization regimen is imperative. Mucosal barriers are the first line of defense against many pathogens and, as such, we looked to their biology for strategies to improve vaccine delivery. Interleukin-22 (IL-22) is a key cytokine in both healthy and inflamed mucosal tissues. IL-22 promotes epithelial cell proliferation and inhibits apoptosis, upregulates mucin and antimicrobial peptides, all of which promote mucosal barrier integrity. In this study, we find that IL-22 impairs the development of a T cell response during mucosal immunization. Compared to wild-type control mice, IL-22 deficient mice had increased antigen-specific CD4 T cell responses to intrarectal immunization using a protein and cholera toxin adjuvant vaccine. When immunized systemically with the same protein antigen adsorbed to alum, no differences in the CD4 T cell response between wild-type and IL-22 deficient mice were detected. This suggests that transiently inhibiting IL-22 during mucosal vaccination could enhance T cell responses. The broad-applicability of this proposed approach would allow for improvement of many existing mucosal vaccine regimens and have positive implications in the development of more efficacious mucosal vaccines.
in vivo CD4+ T cell depletion
Moynihan, K. D., et al (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200.
PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo CD4+ T cell depletion
Flow Cytometry
Liu, G., et al (2015). "IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4+ T Cells and IFN-gamma" PLoS Pathog 11(7): e1005065.
PubMed
African trypanosomes are extracellular protozoan parasites causing a chronic debilitating disease associated with a persistent inflammatory response. Maintaining the balance of the inflammatory response via downregulation of activation of M1-type myeloid cells was previously shown to be crucial to allow prolonged survival. Here we demonstrate that infection with African trypanosomes of IL-27 receptor-deficient (IL-27R-/-) mice results in severe liver immunopathology and dramatically reduced survival as compared to wild-type mice. This coincides with the development of an exacerbated Th1-mediated immune response with overactivation of CD4+ T cells and strongly enhanced production of inflammatory cytokines including IFN-gamma. What is important is that IL-10 production was not impaired in infected IL-27R-/- mice. Depletion of CD4+ T cells in infected IL-27R-/- mice resulted in a dramatically reduced production of IFN-gamma, preventing the early mortality of infected IL-27R-/- mice. This was accompanied by a significantly reduced inflammatory response and a major amelioration of liver pathology. These results could be mimicked by treating IL-27R-/- mice with a neutralizing anti-IFN-gamma antibody. Thus, our data identify IL-27 signaling as a novel pathway to prevent early mortality via inhibiting hyperactivation of CD4+ Th1 cells and their excessive secretion of IFN-gamma during infection with African trypanosomes. These data are the first to demonstrate the essential role of IL-27 signaling in regulating immune responses to extracellular protozoan infections.
in vivo CD4+ T cell depletion
Zander, R. A., et al (2015). "PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity" Cell Host Microbe 17(5): 628-641.
PubMed
The differentiation and protective capacity of Plasmodium-specific T cells are regulated by both positive and negative signals during malaria, but the molecular and cellular details remain poorly defined. Here we show that malaria patients and Plasmodium-infected rodents exhibit atypical expression of the co-stimulatory receptor OX40 on CD4 T cells and that therapeutic enhancement of OX40 signaling enhances helper CD4 T cell activity, humoral immunity, and parasite clearance in rodents. However, these beneficial effects of OX40 signaling are abrogated following coordinate blockade of PD-1 co-inhibitory pathways, which are also upregulated during malaria and associated with elevated parasitemia. Co-administration of biologics blocking PD-1 and promoting OX40 signaling induces excessive interferon-gamma that directly limits helper T cell-mediated support of humoral immunity and decreases parasite control. Our results show that targeting OX40 can enhance Plasmodium control and that crosstalk between co-inhibitory and co-stimulatory pathways in pathogen-specific CD4 T cells can impact pathogen clearance.
in vivo CD4+ T cell depletion
Kim, J., et al (2015). "Memory programming in CD8(+) T-cell differentiation is intrinsic and is not determined by CD4 help" Nat Commun 6: 7994.
PubMed
CD8(+) T cells activated without CD4(+) T-cell help are impaired in memory expansion. To understand the underlying cellular mechanism, here we track the dynamics of helper-deficient CD8(+) T-cell response to a minor histocompatibility antigen by phenotypic and in vivo imaging analyses. Helper-deficient CD8(+) T cells show reduced burst expansion, rapid peripheral egress, delayed antigen clearance and continuous activation, and are eventually exhausted. Contrary to the general consensus that CD4 help encodes memory programmes in CD8(+) T cells and helper-deficient CD8(+) T cells are abortive, these cells can differentiate into effectors and memory precursors. Importantly, accelerating antigen clearance or simply increasing the burst effector size enables generation of memory cells by CD8(+) T cells, regardless of CD4 help. These results suggest that the memory programme is CD8(+) T-cell-intrinsic, and provide insight into the role of CD4 help in CD8(+) T-cell responses.
in vivo CD4+ T cell depletion
Guo, L., et al (2015). "Innate immunological function of TH2 cells in vivo" Nat Immunol 16(10): 1051-1059.
PubMed
Type 2 helper T cells (TH2 cells) produce interleukin 13 (IL-13) when stimulated by papain or house dust mite extract (HDM) and induce eosinophilic inflammation. This innate response is dependent on IL-33 but not T cell antigen receptors (TCRs). While type 2 innate lymphoid cells (ILC2 cells) are the dominant innate producers of IL-13 in naive mice, we found here that helminth-infected mice had more TH2 cells compared to uninfected mice, and thes e cells became major mediators of innate type 2 responses. TH2 cells made important contributions to HDM-induced antigen-nonspecific eosinophilic inflammation and protected mice recovering from infection with Ascaris suum against subsequent infection with the phylogenetically distant nematode Nippostrongylus brasiliensis. Our findings reveal a previously unappreciated role for effector TH2 cells during TCR-independent innate-like immune responses.
in vivo CD4+ T cell depletion
Christensen, A. D., et al (2015). "Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells" Clin Exp Immunol 179(3): 485-499.
PubMed
Regulatory T cells (Tregs ) are known to play an immunosuppressive role in the response of contact hypersensitivity (CHS), but neither the dynamics of Tregs during the CHS response nor the exaggerated inflammatory response after depletion of Tregs has been characterized in detail. In this study we show that the number of Tregs in the challenged tissue peak at the same time as the ear-swelling reaches its maximum on day 1 after challenge, whereas the number of Tregs in the draining lymph nodes peaks at day 2. As expected, depletion of Tregs by injection of a monoclonal antibody to CD25 prior to sensitization led to a prolonged and sustained inflammatory response which was dependent upon CD8 T cells, and co-stimulatory blockade with cytotoxic T lymphocyte antigen-4-immunoglobulin (CTLA-4-Ig) suppressed the exaggerated inflammation. In contrast, blockade of the interleukin (IL)-10-receptor (IL-10R) did not further increase the exaggerated inflammatory response in the Treg -depleted mice. In the absence of Tregs , the response changed from a mainly acute reaction with heavy infiltration of neutrophils to a sustained response with more chronic characteristics (fewer neutrophils and dominated by macrophages). Furthermore, depletion of Tregs enhanced the release of cytokines and chemokines locally in the inflamed ear and augmented serum levels of the systemic inflammatory mediators serum amyloid (SAP) and haptoglobin early in the response.
Evans, E. E., et al (2015). "Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies" Cancer Immunol Res 3(6): 689-701.
PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
in vivo CD4+ T cell depletion
Vanpouille-Box, C., et al (2015). "TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity" Cancer Res 75(11): 2232-2242.
PubMed
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T-cell activity in patients who naturally develop them. Eliciting T-cell responses to a patient’s individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear whether it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFbeta activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here, we show that antibody-mediated TGFbeta neutralization during radiation therapy effectively generates CD8(+) T-cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and nonirradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNgamma and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFbeta blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection, resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFbeta blockade. Thus, TGFbeta is a fundamental regulator of radiation therapy’s ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFbeta neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
in vivo CD4+ T cell depletion
Church, S. E., et al (2014). "Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells" Eur J Immunol 44(1): 69-79.
PubMed
Immunotherapies that augment antitumor T cells have had recent success for treating patients with cancer. Here we examined whether tumor-specific CD4(+) T cells enhance CD8(+) T-cell adoptive immunotherapy in a lymphopenic environment. Our model employed physiological doses of tyrosinase-related protein 1-specific CD4(+) transgenic T cells-CD4(+) T cells and pmel-CD8(+) T cells that when transferred individually were subtherapeutic; however, when transferred together provided significant (p
in vivo CD4+ T cell depletion
Krupnick, A. S., et al (2014). "Central memory CD8+ T lymphocytes mediate lung allograft acceptance" J Clin Invest 124(3): 1130-1143.
PubMed
Memory T lymphocytes are commonly viewed as a major barrier for long-term survival of organ allografts and are thought to accelerate rejection responses due to their rapid infiltration into allografts, low threshold for activation, and ability to produce inflammatory mediators. Because memory T cells are usually associated with rejection, preclinical protocols have been developed to target this population in transplant recipients. Here, using a murine model, we found that costimulatory blockade-mediated lung allograft acceptance depended on the rapid infiltration of the graft by central memory CD8+ T cells (CD44(hi)CD62L(hi)CCR7+). Chemokine receptor signaling and alloantigen recognition were required for trafficking of these memory T cells to lung allografts. Intravital 2-photon imaging revealed that CCR7 expression on CD8+ T cells was critical for formation of stable synapses with antigen-presenting cells, resulting in IFN-gamma production, which induced NO and downregulated alloimmune responses. Thus, we describe a critical role for CD8+ central memory T cells in lung allograft acceptance and highlight the need for tailored approaches for tolerance induction in the lung.
in vivo CD4+ T cell depletion
Xin, L., et al (2014). "Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2" Proc Natl Acad Sci U S A 111(29): 10672-10677.
PubMed
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4(+) T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4(+) T cells with commensal microbe specificity.
in vivo CD4+ T cell depletion
Flow Cytometry
Uddin, M. N., et al (2014). "TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma" J Immunol 192(4): 1946-1953.
PubMed
Using several tumor models, we demonstrate that mice deficient in Bcl11b in T cells, although having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared with wild-type mice. Bcl11b(-/-) CD4(+) T cells, with elevated TNF-alpha levels, but not the Bcl11b(-/-) CD8(+) T cells, were required for the reduced tumor burden, as were NK1.1(+) cells, found in increased numbers in Bcl11b(F/F)/CD4-Cre mice. Among NK1.1(+) cells, the NK cell population was predominant in number and was the only population displaying elevated granzyme B levels and increased degranulation, although not increased proliferation. Although the number of myeloid-derived suppressor cells was increased in the lungs with metastatic tumors of Bcl11b(F/F)/CD4-Cre mice, their arginase-1 levels were severely reduced. The increase in NK cell and myeloid-derived suppressor cell numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung, and increased hematopoiesis in Bcl11b(F/F)/CD4-Cre mice were all dependent on TNF-alpha. Moreover, TNF-alpha treatment of wild-type mice also reduced the tumor burden and increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNF-alpha of lineage-negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNF-alpha in promoting hematopoiesis. These studies reveal a novel role for TNF-alpha in the antitumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
in vivo CD4+ T cell depletion
Hervieu, A., et al (2013). "Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth" J Invest Dermatol 133(2): 499-508.
PubMed
Dacarbazine (DTIC) is a cytotoxic drug widely used for melanoma treatment. However, the putative contribution of anticancer immune responses in the efficacy of DTIC has not been evaluated. By testing how DTIC affects host immune responses to cancer in a mouse model of melanoma, we unexpectedly found that both natural killer (NK) and CD8(+) T cells were indispensable for DTIC therapeutic effect. Although DTIC did not directly affect immune cells, it triggered the upregulation of NKG2D ligands on tumor cells, leading to NK cell activation and IFNgamma secretion in mice and humans. NK cell-derived IFNgamma subsequently favored upregulation of major histocompatibility complex class I molecules on tumor cells, rendering them sensitive to cytotoxic CD8(+) T cells. Accordingly, DTIC markedly enhanced cytotoxic T lymphocyte antigen 4 inhibition efficacy in vivo in an NK-dependent manner. These results underscore the immunogenic properties of DTIC and provide a rationale to combine DTIC with immunotherapeutic agents that relieve immunosuppression in vivo.
in vivo CD4+ T cell depletion
Flow Cytometry
Dai, M., et al (2013). "Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation" J Immunother 36(4): 248-257.
PubMed
40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.”}” data-sheets-userformat=”{“2″:14851,”3”:{“1″:0},”4”:{“1″:2,”2″:16777215},”12″:0,”14”:{“1″:2,”2″:1521491},”15″:”Roboto, sans-serif”,”16″:12}”>Mice with intraperitoneal ID8 ovarian carcinoma or subcutaneous SW1 melanoma were injected with monoclonal antibodies (mAbs) to CD137PD-1CTLA4 7-15 days after tumor initiation. Survival of mice with ID8 tumors tripled and >40% of mice with SW1 tumors remained healthy >150 days after last treatment and are probably cured. Therapeutic efficacy was associated with a systemic immune response with memory and antigen specificity, required CD4 cells and involved CD8 cells and NK cells to a less extent. The 3 mAb combination significantly decreased CD19 cells at tumor sites, increased IFN-gamma and TNF-alpha producing CD4 and CD8 T cells and mature CD86 dendritic cells (DC), and it increased the ratios of effector CD4 and CD8 T cells to CD4Foxp3 regulatory T (Treg) cells and to CD11bGr-1 myeloid suppressor cells (MDSC). This is consistent with shifting the tumor microenvironment from an immunosuppressive Th2 to an immunostimulatory Th1 type and is further supported by PCR data. Adding an anti-CD19 mAb to the 3 mAb combination in the SW1 model further increased therapeutic efficacy. Data from ongoing experiments show that intratumoral injection of a combination of mAbs to CD137PD-1CTLA4CD19 can induce complete regression and dramatically prolong survival also in the TC1 carcinoma and B16 melanoma models, suggesting that the approach has general validity.
in vivo CD4+ T cell depletion
Butler, N. S., et al (2012). "Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection" Nat Immunol 13(2): 188-195.
PubMed
Infection of erythrocytes with Plasmodium species induces clinical malaria. Parasite-specific CD4(+) T cells correlate with lower parasite burdens and severity of human malaria and are needed to control blood-stage infection in mice. However, the characteristics of CD4(+) T cells that determine protection or parasite persistence remain unknown. Here we show that infection of humans with Plasmodium falciparum resulted in higher expression of the inhibitory receptor PD-1 associated with T cell dysfunction. In vivo blockade of the PD-1 ligand PD-L1 and the inhibitory receptor LAG-3 restored CD4(+) T cell function, amplified the number of follicular helper T cells and germinal-center B cells and plasmablasts, enhanced protective antibodies and rapidly cleared blood-stage malaria in mice. Thus, chronic malaria drives specific T cell dysfunction, and proper function can be restored by inhibitory therapies to enhance parasite control.
in vivo CD4+ T cell depletion
Flow Cytometry
Krieg, C., et al (2010). "Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells" Proc Natl Acad Sci U S A 107(26): 11906-11911.
PubMed
IL-2 immunotherapy is an attractive treatment option for certain metastatic cancers. However, administration of IL-2 to patients can lead, by ill-defined mechanisms, to toxic adverse effects including severe pulmonary edema. Here, we show that IL-2-induced pulmonary edema is caused by direct interaction of IL-2 with functional IL-2 receptors (IL-2R) on lung endothelial cells in vivo. Treatment of mice with high-dose IL-2 led to efficient expansion of effector immune cells expressing high levels of IL-2Rbetagamma, including CD8(+) T cells and natural killer cells, which resulted in a considerable antitumor response against s.c. and pulmonary B16 melanoma nodules. However, high-dose IL-2 treatment also affected immune cell lineage marker-negative CD31(+) pulmonary endothelial cells via binding to functional alphabetagamma IL-2Rs, expressed at low to intermediate levels on these cells, thus causing pulmonary edema. Notably, IL-2-mediated pulmonary edema was abrogated by a blocking antibody to IL-2Ralpha (CD25), genetic disruption of CD25, or the use of IL-2Rbetagamma-directed IL-2/anti-IL-2 antibody complexes, thereby interfering with IL-2 binding to IL-2Ralphabetagamma(+) pulmonary endothelial cells. Moreover, IL-2/anti-IL-2 antibody complexes led to vigorous activation of IL-2Rbetagamma(+) effector immune cells, which generated a dramatic antitumor response. Thus, IL-2/anti-IL-2 antibody complexes might improve current strategies of IL-2-based tumor immunotherapy.
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
Microbiota-induced T cell plasticity enables immune-mediated tumour control.
In Nature on 1 March 2026 by Najar, T. A., Hao, Y., et al.
PubMed
Therapies that harness the immune system to target and eliminate tumour cells have revolutionized cancer care. Immune checkpoint blockade (ICB), which boosts the anti-tumour immune response by inhibiting negative regulators of T cell activation1-3, is remarkably successful in a subset of cancer patients. Yet a significant proportion do not respond to treatment, emphasizing the need to understand factors influencing the therapeutic efficacy of ICB4-9. The gut microbiota, consisting of trillions of microorganisms residing in the gastrointestinal tract, has emerged as a critical determinant of immune function and response to cancer immunotherapy, with several studies demonstrating association of microbiota composition with clinical response10-16. However, a mechanistic understanding of how gut commensal bacteria influence the efficacy of ICB remains elusive. Here we use a gut commensal microorganism, segmented filamentous bacteria (SFB), which induces an antigen-specific T helper 17 (TH17) cell effector program in the small intestine lamina propria (SILP)17, to investigate how colonization with this microbe affects the efficacy of ICB in restraining distal growth of tumours sharing antigen with SFB. We find that anti-programmed cell death protein 1 (PD-1) treatment effectively inhibits the growth of implanted SFB antigen-expressing melanoma only if mice are colonized with SFB. Through T cell receptor (TCR) clonal lineage tracing, fate mapping and peptide-major histocompatability complex (MHC) tetramer staining, we identify tumour-associated SFB-specific T helper 1 (TH1)-like cells derived from the homeostatic TH17 cells induced by SFB colonization in the SILP. These gut-educated ex-TH17 cells produce high levels of the pro-inflammatory cytokines interferon (IFN)-γ and tumour necrosis factor (TNF) within the tumour microenvironment (TME), enhancing antigen presentation and promoting recruitment, expansion and effector functions of CD8+ tumour-infiltrating cytotoxic lymphocytes and thereby enabling anti-PD-1-mediated tumour control. Conditional ablation of SFB-induced IL-17A+CD4+ T cells, precursors of tumour-associated TH1-like cells, abolishes anti-PD-1-mediated tumour control and markedly impairs tumour-specific CD8+ T cell recruitment and effector function within the TME. Our data, as a proof of principle, define a cellular pathway by which a single, defined intestinal commensal imprints T cell plasticity that potentiates PD-1 blockade, and indicate targeted modulation of the microbiota as a strategy to broaden ICB efficacy.
-
-
-
Immunology and Microbiology
HRS Degradation-Induced Nicotinamide Deficiency in Placental Extracellular Vesicles Triggers Preeclampsia by Disrupting Maternal-Fetal Immune Homeostasis.
In Adv Sci (Weinh) on 1 February 2026 by Fei, H., Lin, Y., et al.
PubMed
Preeclampsia (PE) is closely associated with alterations in placental extracellular vesicles (pEVs), but the mechanisms and their role in PE pathogenesis remain unclear. This study reveals that nicotinamide (NAM) levels in PE-derived pEVs (PE-EVs) are lower than in pEVs from normal pregnancies, correlating with disease severity. Functionally, NAM in pEVs inhibits Th1 differentiation via SIRT1 suppression and Th17 differentiation via macrophages. NAM-deficient pEVs exhibit reduced capacity to inhibit Th1 and Th17 cell differentiation both in vitro and in vivo, leading to PE-like symptoms. NAM is enriched in pEVs compared to placental villous tissue and maternal serum. The lower NAM in PE-EVs is due to decreased hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) expression in trophoblasts, which loads NAM into the cargo of multivesicular bodies (MVBs) via binding to the tryptophan-115 residue of HRS. Furthermore, the reduction of HRS in PE trophoblasts results from ubiquitination and degradation by elevated HSP27. Collectively, these findings indicate that elevated HSP27 in PE trophoblasts leads to the degradation of HRS, a reduction in pEV NAM levels, and diminished Th1 and Th17 inhibitory effects, thereby contributing to the development of PE.
-
-
-
Immunology and Microbiology
-
Cell Biology
-
Cancer Research
SLC2A1+ tumour-associated macrophages spatially control CD8+ T cell function and drive resistance to immunotherapy in non-small-cell lung cancer.
In Nat Cell Biol on 1 February 2026 by Wang, L., Chu, H., et al.
PubMed
Tumour-associated macrophages (TAMs) contribute to immune checkpoint blockade resistance, but their impact on intratumoural CD8⁺ T cell distribution remains unclear. Here we show that the expression of the glucose transporter SLC2A1 is spatially negatively correlated with CD8⁺ T cell distribution in both non-small-cell lung cancer (NSCLC) biopsies and murine tumour models. Tumour cell-specific Slc2a1 knockdown fails to reproduce the therapeutic benefit of SLC2A1 inhibition, whereas TAM-specific deletion of Slc2a1 suppresses tumour growth by enhancing the spatial homogeneity and effector function of intratumoural CD8⁺ T cells, thereby improving αPD-L1 efficacy. Spatial profiling of NSCLC specimens further revealed that SLC2A1⁺ TAM-enriched regions exhibit reduced CD8⁺ T cell density, and spatial proximity between these populations predicts resistance to αPD-(L)1 therapy. These findings identify SLC2A1⁺ TAMs as drivers of spatial CD8⁺ T cell exclusion and highlight TAM-specific SLC2A1 as a therapeutic target to overcome immune checkpoint blockade resistance in NSCLC.
-
-
-
Cancer Research
-
Immunology and Microbiology
Cathepsin-D-mediated MHC class I degradation contributes to immune evasion in colorectal cancer.
In Cell Rep Med on 20 January 2026 by Zhan, W., Fu, Y., et al.
PubMed
Microsatellite stable (MSS) colorectal cancer (CRC) is often considered a "cold" tumor with limited response to programmed death-1 (PD-1) antibody monotherapy. The mechanisms underlying its intrinsic resistance to immunotherapy remain unclear. Here, we show that cathepsin D (CTSD) is highly expressed in MSS CRC and contributes significantly to immunotherapy resistance. Mechanistically, CTSD, acting as a protease, interacts with the α2 domain of the major histocompatibility complex (MHC) class I via the light chain of its catalytic domain, promoting MHC class I degradation through lysosomal pathways and impairing its recycling to the cell surface. This mechanism shields tumor cells from cytotoxic T-cell-mediated killing and facilitates immune evasion. Notably, genetic deletion or pharmacological inhibition of CTSD using pepstatin A prevents immune escape and enhances anti-PD-1 efficacy. These findings identify CTSD as a key mediator of immune evasion in MSS CRC and support the development of a combination therapy comprising CTSD inhibition and anti-PD-1 immunotherapy.
-
-
-
Cancer Research
Targeting severe acidity for tumor-activatable Interleukin-2 therapy.
In Cell Rep Med on 20 January 2026 by Feng, Q., Pantoja, R., et al.
PubMed
Interleukin-2 (IL-2) is a cytokine with curative potential in cancer immunotherapy, but its clinical use is limited by a narrow therapeutic window. Traditional strategies such as polarizing receptor binding or fusing IL-2 with Fc (IL-2-Fc) improve pharmacokinetics and immune selectivity, but systemic toxicity remains a key challenge, while covalent prodrug designs may compromise potency and restrict applicability. Here, we present a non-covalent approach using clinically validated ultra pH-sensitive (UPS) polymers to enable tumor-specific IL-2 activation. The UPS5.3/IL-2-Fc nanoparticle remains stable at physiological pH, minimizing receptor binding in normal tissues, but dissociates and restores IL-2 activity in severely acidic tumor environments (pH < 5.3). This pH-triggered activation reduces systemic toxicity, resulting in over 100-fold reduction in circulating interferon-γ and prevention of vascular leak syndrome, while preserving antitumor efficacy. Mechanistically, the protective effect relies on both pH-dependent shielding and macrophage clearance. This bioengineering strategy offers a generalizable framework for immune cytokine therapy.
-
-
-
Immunology and Microbiology
-
Cancer Research
Targeting the N-acetyltransferase 10/DKK2 axis enhances CD8+ T cell antitumor activity in colorectal cancer models.
In J Clin Invest on 16 January 2026 by Li, M., Zhao, X., et al.
PubMed
Despite overexpression of N-acetyltransferase 10 (NAT10) in colorectal cancer (CRC), its immunomodulatory role in the tumor microenvironment remains elusive. Here, we reveal that NAT10 promotes immune evasion through N4-acetylcytosine-dependent (ac4C-dependent) mRNA stabilization. Using syngeneic mouse models (MC38/CT-26), intestinal epithelial-cell specific Nat10 conditional KO (Nat10cKO) mice, patient-derived organoids, and clinical specimens, we show that Nat10 ablation enhanced CD8+ T cell-mediated antitumor immunity. Single-cell RNA-seq revealed increased cytotoxic CD8+ T cell infiltration in Nat10cKO tumors, which was corroborated by the inverse correlation of tumoral NAT10 expression and CD8+ T cell number in clinical specimens. Multi-omics integration analysis identified DKK2 as the predominant NAT10-regulated transcript. NAT10 stabilized DKK2 mRNA via ac4C modification, leading to high expression of the DKK2 protein. Secreted DKK2 engaged LRP6 receptors to activate AKT-mTOR signaling, inducing cholesterol accumulation in CD8+ T cells and impairing their cytotoxicity. Pharmacological NAT10 inhibition (Remodelin treatment) or DKK2 neutralization restored CD8+ T cell function and synergized with anti-PD-1 therapy. Our findings establish the NAT10/DKK2/LRP6/AKT-mTOR/cholesterol axis as a critical regulator of CD8+ T cell dysfunction in CRC, positioning NAT10/DKK2 as a potential target to enhance immunotherapy efficacy.
-
-
-
Cancer Research
-
Immunology and Microbiology
Predominant mutated non-canonical tumor-specific antigens identified by proteogenomics demonstrate immunogenicity and tumor suppression in CRC.
In Cell Genom on 14 January 2026 by Xiang, H., Guan, X., et al.
PubMed
Tumor-specific antigens (TSAs) are crucial for activating T cells against cancer, but traditional discovery methods focusing on exonic mutations overlook non-canonical TSAs from non-coding regions. We employed an integrative proteogenomic strategy combining whole-genome and RNA sequencing with immunoprecipitation mass spectrometry to comprehensively explore TSA generation in colorectal cancer patients. Analysis of 10 paired tumor samples identified 96 mutated major histocompatibility complex class I-presented neo-epitopes, with 80.21% originating from non-coding regions. In hypermutated tumors with high mutational burden, neo-epitopes predominantly arose from intergenic and intronic areas, while in non-hypermutated tumors with low mutational burden, they mainly stemmed from coding variations and alternative splicing events. Functional validation in mouse models demonstrated that mutated non-canonical neo-epitopes effectively activated CD8+ T cells and significantly suppressed tumor growth. These findings underscore the importance of considering the entire genomic landscape in TSA discovery, suggesting new avenues for personalized immunotherapy.
-
-
-
Immunology and Microbiology
-
Cancer Research
RAS(ON) Multiselective Inhibition Drives Antitumor Immunity in Preclinical Models of NRAS-Mutant Melanoma.
In Cancer Immunol Res on 8 January 2026 by Carvalho, L. A., Tovbis Shifrin, N., et al.
PubMed
Targeted therapies for NRAS-mutant melanoma remain an unmet clinical need. In this study, we demonstrate that RMC-7977, a preclinical RAS(ON) multiselective inhibitor representative of the investigational agent daraxonrasib (RMC-6236), was able to elicit potent antitumor immune responses across multiple NRAS-mutant melanoma models. Treatment with RMC-7977 led to rapid tumor regressions driven by inhibition of MAPK signaling, upregulation of MHC and PD-L1 proteins, and enhanced infiltration of CD4+ and CD8+ T cells. Complete responses were dependent on adaptive immunity, as both CD4+ and CD8+ T cells were essential for extended survival. Resistance to treatment was marked by reduced T-cell infiltration, loss of MHC class I expression, and expansion of myeloid-derived suppressor cells. Combining RMC-7977 with anti-PD-1 boosted cytotoxic T-cell infiltration, reprogrammed myeloid cells toward an antigen-presenting phenotype, and improved survival in models resistant to PD-1 blockade. Consistent with these preclinical data, objective clinical responses were observed in two patients with NRAS-mutant melanoma treated with daraxonrasib in an ongoing phase I/Ib clinical trial. Together, these data support the continued clinical evaluation of RAS(ON) multiselective inhibitors for the treatment of NRAS-mutant melanoma.
-
-
-
Cancer Research
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TGFβ induces an atypical EMT to evade immune mechanosurveillance in lung adenocarcinoma dormant metastasis.
In Nat Cancer on 1 January 2026 by Wang, Z., Elbanna, Y., et al.
PubMed
Different forms of epithelial-to-mesenchymal transition (EMT) manifest during tumor progression. Little is known about the mechanistic basis and functional role of these distinct EMTs. We explored this question in lung adenocarcinoma (LUAD) primitive progenitors, which are competent to enter dormancy in response to transforming growth factor-β (TGFβ) upon metastatic dissemination. The TGFβ response in these cells includes growth arrest and a full EMT that subsequently transitions into an atypical mesenchymal state of round morphology and lacking actin stress fibers. TGFβ drives this transition by inducing expression of the actin depolymerizing protein gelsolin, which converts a migratory, stress-fiber-rich phenotype into a cortical actin-rich, spheroidal state. This transition lowers the biomechanical stiffness of metastatic progenitors and protects them from killing by cytotoxic lymphocytes. Gelsolin-deficient LUAD progenitors can enter dormancy but succumb to immune surveillance. Thus, quiescent LUAD metastatic progenitors undergo an atypical EMT to avert immune surveillance during TGFβ-driven metastatic dormancy.
-
-
-
Immunology and Microbiology
-
Cancer Research
CCL19-armed recombinant influenza virus inhibited colorectal cancer growth by remodeling tumor microenvironment.
In iScience on 19 December 2025 by Ou, X., Xia, Y., et al.
PubMed
The "cold" tumor microenvironment (TME) impaired colorectal cancer (CRC) immunotherapy efficacy, while oncolytic viruses (OVs) could "heat" TME and stimulate anti-tumor immunity. Here, it rescued rPR8-CCL19, a recombinant oncolytic influenza virus expressing CCL19, using reverse genetics technology. Gene sequencing and transmission electron microscopy confirmed its genetic stability during serial passaging. Hemagglutination assay, ELISA, transwell, real-time cell analysis (RTCA), and apoptosis detection demonstrated that it selectively infected CRC cells, expressed CCL19 with the function of chemotaxis and activation on immune cells, and exerted killing of significant CRC cells. In syngeneic CRC mouse models, it effectively inhibited tumor growth and metastasis, prolonging survival. By enhancing immune cell infiltration, it remodeled the TME, thereby inducing systemic anti-tumor immunity and even immune memory, without causing severe pathological damage. This study confirmed rPR8-CCL19 as a promising CRC immunotherapy for further exploration.
-
-
-
Immunology and Microbiology
Next-generation Candida albicans vaccine VXV-01 containing recombinant Als3p and Hyr1p antigens for invasive Candida infections.
In Sci Rep on 15 December 2025 by Singh, S., Youssef, E. G., et al.
PubMed
Candida species, including Candida albicans and Candida auris, represent a growing public health concern due to their increasing prevalence and resistance to antifungal agents. C. albicans is known for causing both superficial and invasive infections, while C. auris is a newly emerged, multidrug-resistant pathogen responsible for severe hospital outbreaks with a high mortality rate of ~ 60% in bloodstream infections. Vaccine candidates targeting C. albicans hyphal cell wall proteins Als3p and Hyr1p have shown protective efficacy in mice. NDV-3A, an alum-formulated Als3p-based vaccine, protected against recurrent vulvovaginal candidiasis in women. We earlier showed that both Als3p and Hyr1p have orthologs in C. auris, and that the NDV-3A vaccine, alongside an anti-Hyr1p monoclonal antibody, protected mice from multidrug resistant C. auris candidemia. Here, we optimized VXV-01, an Als3p and Hyr1p dual antigen vaccine formulated with the clinical-stage adjuvant CAF01, demonstrating robust immunity and CD4 T cell-dependent protection against lethal C. albicans and C. auris. The VXV-01 vaccine did not antagonize antifungal drug therapy and showed higher overall mouse survival than mice receiving the vaccine or antifungal drug alone, albeit this difference did not reach statistical significance. This study highlights the potential of VXV-01 in providing durable protective immunity against hematogenously disseminated C. albicans and C. auris and mucosal C. albicans infections.
-
-
Lineage tracing of both quiescent G0 and active Hoxb5+ LT-HSCs that actively contribute to homeostatic mouse hematopoiesis.
In Proc Natl Acad Sci U S A on 9 December 2025 by Xiang, J., Almeida, L., et al.
PubMed
Studying the lineage commitment and differentiation potential of long-term hematopoietic stem cells (LT-HSCs) is important to understand the dynamics of hematopoiesis. A central question concerns which hematopoietic stem and progenitor cell populations are responsible for sustaining steady-state hematopoiesis in vivo without conditioning. Noninvasive HSC fate-mapping strategies to address this question require specific labeling of LT-HSCs only. In this study, we selectively labeled a subset of Hoxb5+ LT-HSCs-excluding short-term HSCs (ST-HSCs) and multipotent progenitors (MPPs)-to track the progeny of these cells. Hoxb5+ LT-HSCs comprise ~1 in 100,000 bone marrow cells. MPPs were not labeled until several months post-induction, indicating their derivation from LT-HSCs. At no time were MPPs labeled and LT-HSCs not, consistent with the origin and maintenance of MPPs from LT-HSCs. Hoxb5+ LT-HSCs are the principal contributors to steady-state in situ hematopoiesis, but only a fraction of LT-HSCs were labeled by the Cre/LoxP conversion to a lineage-tracing color. We tested whether quiescent HSCs could have incised the DNA at loxp sites, but did not finish the rearrangement. Analysis of phosphorylated H2AX (γ-H2AX) revealed that quiescent LT-HSCs retain Cre/LoxP-induced DNA incisions, which are repaired upon cell cycle entry, leading to the appearance of newly labeled LT-HSCs at later time points, mainly of the myeloid-biased HSC. Moreover, most LT-HSCs exhibit marked expansion in response to hematopoietic stress. With the age-related shift of blood formation from balanced to myeloid biased, the myeloid-biased HSCs expand preferentially after 6 mo of tracking.
-
-
Immunology and Microbiology
-
Cancer Research
ERBB2 signaling drives immune cell evasion and resistance against immunotherapy in small cell lung cancer.
In Nat Commun on 9 December 2025 by Meder, L., Orschel, C. I., et al.
PubMed
Small cell lung cancer (SCLC) is characterized by its highly aggressive phenotype and dismal outcome. Despite the benefit of adding immune checkpoint blockade to standard chemotherapy, tumors acquire the ability to evade immunosurveillance and develop resistance. To investigate these underlying mechanisms, we perform high-dimensional profiling of human and murine SCLC specimens. In matched primary and metastatic human samples, we observe MHC-I loss in metastases, highlighting its role in immune evasion. Correspondingly, silencing MHC-I in SCLC cells drastically reduces immune infiltration and promotes metastasis in mice. Using mass spectrometry and phospho-tyrosine kinase analyses, we identify ERBB2 signaling as a suppressor of MHC-I and driver of immune-modulatory transcripts. Mechanistically, genetic and pharmacologic blockade of ERBB2 induces MHC-I in a STING-dependent manner and prevents immune evasion in autochthonous murine SCLC. Strikingly, combining ERBB2 inhibition with anti-PD-1 elicits profound synergistic responses in preclinical models, suggesting this combination for future clinical trials in SCLC patients.
-
-
-
Genetics
-
Immunology and Microbiology
mRNA vaccine expressing enterovirus D68 virus-like particles induces potent neutralizing antibodies and protects against infection.
In Mol Ther Nucleic Acids on 9 December 2025 by Kunishima, Y., Senpuku, K., et al.
PubMed
Enterovirus D68 (EV-D68) causes respiratory illness in children. It also causes severe paralysis called acute flaccid myelitis (AFM), which has become a global health threat. Here, we generated an mRNA vaccine expressing virus-like particles (VLPs) of EV-D68. We found that the mRNA vaccine elicited potent neutralizing antibodies against EV-D68 in the blood, and the neutralizing titer was superior to that of the inactivated whole virion (IWV) vaccine. The mRNA vaccine showed protective effects against intranasal challenge with EV-D68, and antisera from the vaccinated mice prevented the paralysis caused by EV-D68 infection in neonatal mice. Moreover, the mRNA vaccine induced neutralizing antibodies in the respiratory tract, which is the entry site for EV-D68. Additionally, it attenuated infection with coxsackievirus B3 (CVB3), which belongs to another enterovirus group, via CD8+ T cell responses. In conclusion, our results suggest that this mRNA vaccine is a promising candidate for EV-D68 prevention.
-
-
-
Cancer Research
-
Immunology and Microbiology
ATOR-4066, a Bispecific Antibody Targeting CD40 and CEACAM5, Induces Strong Myeloid and T Cell-Dependent Tumor Immunity and Synergizes with PD-1 Blockade.
In Cancer Immunol Res on 2 December 2025 by Andersson, H., Uddbäck, I., et al.
PubMed
Despite recent progress within the field of immuno-oncology, immune suppression in the tumor microenvironment, defective antigen presentation, and low levels of tumor-specific T cells are key limitations of current cancer immunotherapies. CD40-targeting immunotherapies hold promise for addressing these limitations across solid tumors. In this study, we describe ATOR-4066, a bispecific antibody that targets CD40 and CEACAM5, developed using the Neo-X-Prime platform. ATOR-4066 showed potent CEACAM5-dependent activation in vitro, with an ability to activate intratumoral immune cells from patient-derived material. In vivo, ATOR-4066 induced superior antitumor activity compared with a CD40 mAb in MC38-carcinoembryonic antigen tumors and cured mice with well-established tumors with heterogeneous CEACAM5 expression. Using RNA sequencing, flow cytometry, and cytokine analysis, we showed that ATOR-4066 promoted immune cell trafficking to tumors and activated both myeloid cells and T cells within the tumor microenvironment, with limited immune activation in the periphery. ATOR-4066 initially induced a T cell-independent antitumor response, yet we found that a functional T-cell response was critical for long-term tumor control and immunity directed to tumor antigens other than CEACAM5. Finally, we demonstrated that ATOR-4066 synergized with PD-1 blockade in vitro. In conclusion, these data provide mechanistic evidence for the proposed mode of action and support further development of ATOR-4066 in CEACAM5-expressing cancers.
-
-
-
Immunology and Microbiology
Virus envelope glycoprotein targeting bispecific T cell engager protects mice from lethal severe fever with thrombocytopenia virus infection.
In Cell Rep Med on 18 November 2025 by Peng, X., Wang, W., et al.
PubMed
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging hemorrhagic fever disease caused by the SFTS virus (SFTSV). Despite pandemic concerns arising from repeated instances of human-to-human transmission and a high fatality rate, effective anti-SFTSV interventions remain unavailable. Here, utilizing single-cell RNA sequencing (scRNA-seq) and flow cytometry data, we revealed that the deficiency and dysfunction states of T cells, particularly the impaired cytotoxicity and exhausted state of CD4+ T cells, were significantly associated with lethal consequences in SFTS patients. Using an infectious mouse model, we further observed that depletion of CD4+ T and CD8+ T cells was related to elevated viremia and increased fatality rates in SFTSV-infected mice. Accordingly, we designed virus envelope glycoprotein-targeting bispecific T cell engager (BiTE) antibodies to redirect T cells to eliminate SFTSV-infected cells, effectively rescuing mice from lethal SFTSV infection. Collectively, Gn-targeted BiTEs hold potential as a therapeutic option for treating SFTS.
-
-
-
Cancer Research
A bispecific antibody targeting PD-L1/TNFR2 increases tumor targeting and enhances antitumor efficacy in colorectal cancer.
In J Immunother Cancer on 13 November 2025 by Kang, X., Qian, P., et al.
PubMed
Immune checkpoint inhibitors (ICIs) have shown limited efficacy in colorectal cancer (CRC), largely due to immunosuppressive tumor microenvironment (TME) including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Additionally, the off-target effects of ICIs can reduce drug accumulation in tumor tissues and lead to immune-related adverse events, further compromising their clinical utility.
-
-
-
Immunology and Microbiology
Engagement of the T cell receptor against an oncolytic virus generates a population of hypereffector CAR T cells with potent antitumor activity
In Research Square on 12 November 2025 by Vile, R., Liseth, O., et al.
-
-
-
Immunology and Microbiology
Trained ILC2 prevent IL-17-associated lung injury during helminth infection through a serotonin-dependent mechanism
In bioRxiv on 4 November 2025 by Femoe, U. M., Musaigwa, F., et al.
-
-
-
Immunology and Microbiology
Salmonella-superspreader hosts require gut regulatory T cells to maintain a disease-tolerant state.
In J Exp Med on 3 November 2025 by Di Luccia, B., Massis, L. M., et al.
PubMed
Host-pathogen interactions involve two critical strategies: resistance, whereby hosts clear invading microbes, and tolerance, whereby hosts carry high pathogen burden asymptomatically. Here, we investigate mechanisms by which Salmonella-superspreader (SSP) hosts maintain an asymptomatic state during chronic infection. We found that regulatory T cells (Tregs) are essential for this disease-tolerant state, limiting intestinal immunopathology and enabling SSP hosts to thrive, while facilitating Salmonella transmission. Treg depletion in SSP mice resulted in decreased survival, heightened gut inflammation, and impairment of the intestinal barrier, without affecting Salmonella persistence. Colonic Tregs from SSP mice exhibited a unique transcriptomic profile characterized by the upregulation of type 1 inflammatory genes, including the transcription factor T-bet. In the absence of Tregs, we observed robust expansion of cytotoxic CD4+ T cells, with CD4+ T cell depletion restoring homeostasis. These results uncover a critical host strategy to establish disease tolerance during chronic enteric infection, providing novel insights into mucosal responses to persistent pathogens and chronic intestinal inflammation.
-