How purity, validation, and rigorous controls safeguard data integrity and accelerate discovery
Introduction
Functional antibodies are critical for exploring disease pathways, testing therapeutic strategies, and building translational models. Their power lies in an ability to modulate biology directly, and antibody quality therefore determines the reliability of experimental outcomes. Premium antibodies are distinguished by rigorous characterization, high purity, and consistent performance. Choosing well-validated reagents reduces confounding variables, strengthens reproducibility, and saves valuable time.
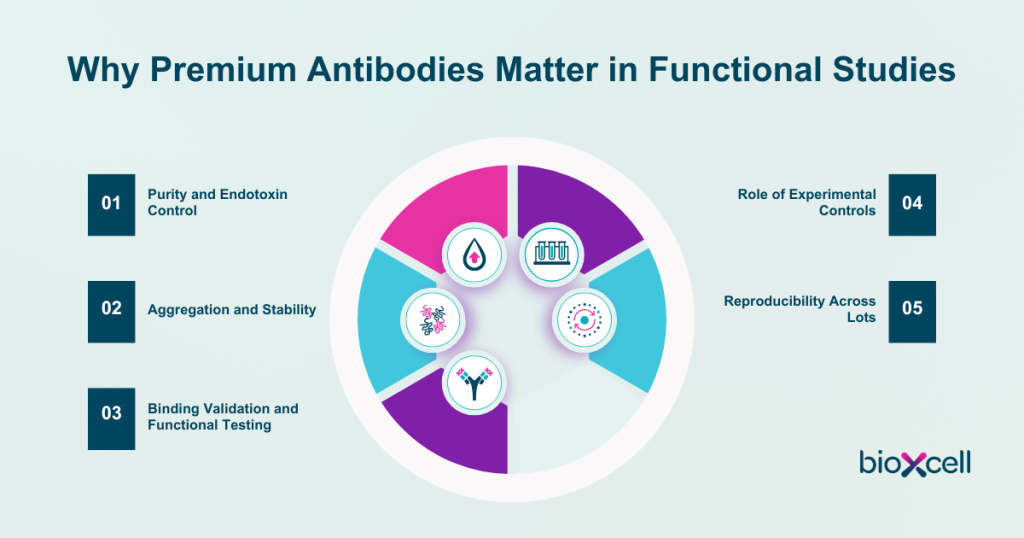
Purity and Endotoxin Control
Reproducible purity is the gold standard of functional antibodies. Even trace contaminants can alter immune readouts or compromise cell viability. Endotoxin, a byproduct of bacterial expression systems, can provoke cytokine release and obscure the true activity of an antibody¹. Premium antibodies are formulated to be ultra pure with low endotoxin levels, ensuring that immune activation reflects the intended biology rather than hidden contaminants.
For researchers working in sensitive preclinical assays, premium-grade functional antibodies provide an essential safeguard: comprehensive murine pathogen testing, which protects both study integrity and animal health. InVivoPlus™ antibodies from Bio X Cell are routinely screened for a broad panel of common murine pathogens, including:
Mycoplasma species:
- M. pulmonis, M. arginini, M. fermentans, M. hominis, M. hyorhinis, M. orale, M. pirum, M. salivarium, M. agassizii, M. cynos
- Murine viruses:
- Murine norovirus (MNV)
- Murine parvovirus (MPV 1–5)
- Murine minute virus (MMV/MVM)
- Murine hepatitis virus (MHV)
- Murine reovirus (REO types 1–3)
- Lymphocytic choriomeningitis virus (LCMV)
- Lactate dehydrogenase–elevating virus (LDV)
- Murine rotavirus (MRV/EDIM)
- Theiler’s murine encephalomyelitis virus (TMEV)
- Ectromelia virus (ECTRO)
- Hantavirus (HANTA)
- Polyomavirus (POLY)
- Murine adenovirus (MAD1, MAD2)
- Sendai virus (SEND)
- Pneumonia virus of mice (PVM)
- Murine cytomegalovirus (MCMV 1, 2)
- K virus
This level of screening ensures that Bio X Cell’s InVivoPlus™ portfolio meets the most stringent standards for pathogen safety, supporting reproducible results in the most sensitive in vivo and translational studies.
Sidebar: Endotoxin as a Hidden Variable
Endotoxin contamination is measured in endotoxin units. Levels above about 1 EU/mg can elicit immune responses in sensitive in vivo systems, adding background activity that can mimic or mask functional outcomes².
Takeaway: Low-endotoxin formulations are essential for reliable in vivo studies.
Aggregation and Stability
Aggregated antibodies can artificially cluster cell-surface receptors and exaggerate signaling responses³. Aggregation may also accelerate clearance or alter biodistribution in animal models. Premium antibodies are tested for aggregation and stability to support consistent performance across experiments and timeframes.
Binding Validation and Functional Testing
Antibodies validated for detection are not always appropriate for functional use. Functional studies require reagents that are tested for binding specificity and biological activity in the intended context. This includes confirmation that the antibody binds its native target conformation and, when relevant, that Fc activity contributes as expected. Without functional validation, results can be misleading. For example, antibodies validated only in denatured Western blot applications may fail in receptor signaling assays or
in vivo immune models⁴.
Role of Experimental Controls
Premium antibodies are often accompanied by matched isotype controls. These controls enable researchers to separate target-specific effects from non-specific background signals, which is especially important in Fc receptor–rich environments. Isotype controls paired with high-purity functional antibodies provide a clearer view of the biology being studied.
Reproducibility Across Lots
Variability between production lots has been cited as a contributor to irreproducibility in preclinical research⁵. Premium suppliers address this by providing lot-specific certificates of analysis and maintaining consistency across production runs. For long-term or translational projects, this level of reproducibility reduces experimental risk and increases confidence in results.
Support for Your Studies
Premium antibodies should come with premium support to ensure experimental success and reduce uncertainty around study design. Bio X Cell products have been selected with PhD-level scientific guidance, application-specific validation data, and transparent documentation to help researchers like you design, troubleshoot, and optimize experiments.
Unlike other reagent providers, Bio X Cell is the only life science company offering 100% PhD-level technical support worldwide. The Bio X Cell approach, “for scientists, by scientists”, extends from catalog target selection and antibody validation to QC data review, ensuring that the antigen and clone you choose are the best fit for your experimental question. Whether you need help selecting the right PD-1 clone for your model, interpreting lot-specific QC data, or planning scale-up supply, our expert team reduces uncertainty so you can focus on generating reproducible, impactful results.
The World’s Most Cited Functional Antibodies - Only from Bio X Cell
With nearly 30,000 peer-reviewed citations, Bio X Cell is the only functional antibody supplier able to demonstrate such a robust legacy of consistency, enabling global translational research since 1997. Far from generic clones or commodity reagents, Bio X Cell antibodies are the original, industry-leading standard, delivering unmatched reproducibility, scalability, flexibility, and consistency that researchers worldwide rely on to drive breakthrough discoveries in disease research.
Conclusion
Premium antibodies are not a luxury. They are a necessity for functional research. By focusing on purity, stability, functional validation, robust controls, and reproducibility, researchers can minimize confounders and generate results that reflect underlying biology.
Teaser for Next Blog: The final post in this series explains how to select the right model system for functional antibody studies, from
in vitro organoid/organ-on-chip platforms to
in vivo mouse models.
References
- Petsch D, Anspach FB. Endotoxin removal from protein solutions. J Biotechnol. 2000;76:97–119.
- Gorbet MB, Sefton MV. Endotoxin: The uninvited guest. Biomaterials. 2005;26(34):6811–6817.
- Aricescu AR, et al. Aggregation and endotoxin contamination as critical confounders in antibody studies. MAbs. 2016;8(2):280–285.
- Uhlén M, et al. A proposal for validation of antibodies. Nat Methods. 2016;13(10):823–827.
- Bradbury A, Plückthun A. Reproducibility: Standardize antibodies used in research. Nature. 2015;518:27–29.