InVivoMAb anti-mouse CD28
Product Description
Specifications
| Isotype | Armenian Hamster IgG, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | C57BL/6 mouse T cell lymphoma EL-4 cells |
| Reported Applications | in vitro T cell stimulation/activation |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107628 |
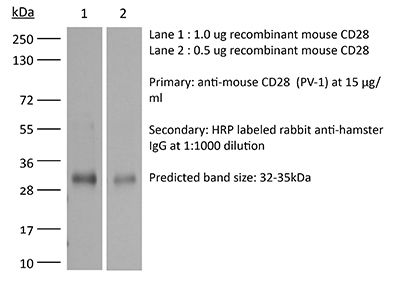
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro IFNγ neutralization
in vitro T cell stimulation/activation
in vitro T cell stimulation/activation
in vitro IL-12 neutralization
Bertin, S., et al (2014). "The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells" Nat Immunol 15(11): 1055-1063.
PubMed
TRPV1 is a Ca(2+)-permeable channel studied mostly as a pain receptor in sensory neurons. However, its role in other cell types is poorly understood. Here we found that TRPV1 was functionally expressed in CD4(+) T cells, where it acted as a non-store-operated Ca(2+) channel and contributed to T cell antigen receptor (TCR)-induced Ca(2+) influx, TCR signaling and T cell activation. In models of T cell-mediated colitis, TRPV1 promoted colitogenic T cell responses and intestinal inflammation. Furthermore, genetic and pharmacological inhibition of TRPV1 in human CD4(+) T cells recapitulated the phenotype of mouse Trpv1(-/-) CD4(+) T cells. Our findings suggest that inhibition of TRPV1 could represent a new therapeutic strategy for restraining proinflammatory T cell responses.
in vitro IL-4 neutralization
in vitro T cell stimulation/activation
Heinemann, C., et al (2014). "IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1" Nat Commun 5: 3770.
PubMed
Central nervous system (CNS) autoimmunity is regulated by the balance of pro-inflammatory cytokines and IL-10. Here we identify the transcriptional regulator Blimp1 as crucial to induce IL-10 in inflammatory T helper cells. Pre-committed Th17 cells respond to IL-27 and IL-12 by upregulating Blimp1 and adopt a Tr-1-like phenotype characterized by IL-10 and IFN-gamma production. Accordingly, Blimp1-deficient effector T cells fail to produce IL-10, and deficiency in Tr-1 cell function leads to uncontrolled Th17 cell-driven CNS pathology without the need to stabilize the Th17 phenotype with IL-23. IL-23 counteracts IL-27 and IL-12-mediated effects on Tr-1-development reinforcing the pro-inflammatory phenotype of Th17 cells. Thus, the balance of IL-23 vs IL-12/IL-27 signals into CD4(+) effector T cells determines whether tissue inflammation is perpetuated or resolves.
in vitro IFNγ neutralization
in vitro T cell stimulation/activation
in vitro T cell stimulation/activation
in vitro IL-12 neutralization
Bertin, S., et al (2014). "The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells" Nat Immunol 15(11): 1055-1063.
PubMed
TRPV1 is a Ca(2+)-permeable channel studied mostly as a pain receptor in sensory neurons. However, its role in other cell types is poorly understood. Here we found that TRPV1 was functionally expressed in CD4(+) T cells, where it acted as a non-store-operated Ca(2+) channel and contributed to T cell antigen receptor (TCR)-induced Ca(2+) influx, TCR signaling and T cell activation. In models of T cell-mediated colitis, TRPV1 promoted colitogenic T cell responses and intestinal inflammation. Furthermore, genetic and pharmacological inhibition of TRPV1 in human CD4(+) T cells recapitulated the phenotype of mouse Trpv1(-/-) CD4(+) T cells. Our findings suggest that inhibition of TRPV1 could represent a new therapeutic strategy for restraining proinflammatory T cell responses.
in vivo IL-17A neutralization
in vitro T cell stimulation/activation
in vitro T cell stimulation/activation
in vivo IL-6 neutralization
Berger, H., et al (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590.
PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vitro IL-4 neutralization
in vitro T cell stimulation/activation
Heinemann, C., et al (2014). "IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1" Nat Commun 5: 3770.
PubMed
Central nervous system (CNS) autoimmunity is regulated by the balance of pro-inflammatory cytokines and IL-10. Here we identify the transcriptional regulator Blimp1 as crucial to induce IL-10 in inflammatory T helper cells. Pre-committed Th17 cells respond to IL-27 and IL-12 by upregulating Blimp1 and adopt a Tr-1-like phenotype characterized by IL-10 and IFN-gamma production. Accordingly, Blimp1-deficient effector T cells fail to produce IL-10, and deficiency in Tr-1 cell function leads to uncontrolled Th17 cell-driven CNS pathology without the need to stabilize the Th17 phenotype with IL-23. IL-23 counteracts IL-27 and IL-12-mediated effects on Tr-1-development reinforcing the pro-inflammatory phenotype of Th17 cells. Thus, the balance of IL-23 vs IL-12/IL-27 signals into CD4(+) effector T cells determines whether tissue inflammation is perpetuated or resolves.
in vivo IL-17A neutralization
in vitro T cell stimulation/activation
in vitro T cell stimulation/activation
in vivo IL-6 neutralization
Berger, H., et al (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590.
PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vitro T cell stimulation/activation
in vivo CD8+ T cell depletion
in vitro T cell stimulation/activation
in vivo IL-9 neutralization
in vivo IL-10 neutralization
Vegran, F., et al (2014). "The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells" Nat Immunol 15(8): 758-766.
PubMed
The TH9 subset of helper T cells was initially shown to contribute to the induction of autoimmune and allergic diseases, but subsequent evidence has suggested that these cells also exert antitumor activities. However, the molecular events that account for their effector properties are elusive. Here we found that the transcription factor IRF1 enhanced the effector function of TH9 cells and dictated their anticancer properties. Under TH9-skewing conditions, interleukin 1beta (IL-1beta) induced phosphorylation of the transcription factor STAT1 and subsequent expression of IRF1, which bound to the promoters of Il9 and Il21 and enhanced secretion of the cytokines IL-9 and IL-21 from TH9 cells. Furthermore, IL-1beta-induced TH9 cells exerted potent anticancer functions in an IRF1- and IL-21-dependent manner. Our findings thus identify IRF1 as a target for controlling the function of TH9 cells.
in vitro T cell stimulation/activation
in vitro T cell stimulation/activation
Huang, Y., et al (2015). "CRK proteins selectively regulate T cell migration into inflamed tissues" J Clin Invest 125(3): 1019-1032.
PubMed
Effector T cell migration into inflamed sites greatly exacerbates tissue destruction and disease severity in inflammatory diseases, including graft-versus-host disease (GVHD). T cell migration into such sites depends heavily on regulated adhesion and migration, but the signaling pathways that coordinate these functions downstream of chemokine receptors are largely unknown. Using conditional knockout mice, we found that T cells lacking the adaptor proteins CRK and CRK-like (CRKL) exhibit reduced integrin-dependent adhesion, chemotaxis, and diapedesis. Moreover, these two closely related proteins exhibited substantial functional redundancy, as ectopic expression of either protein rescued defects in T cells lacking both CRK and CRKL. We determined that CRK proteins coordinate with the RAP guanine nucleotide exchange factor C3G and the adhesion docking molecule CASL to activate the integrin regulatory GTPase RAP1. CRK proteins were required for effector T cell trafficking into sites of inflammation, but not for migration to lymphoid organs. In a murine bone marrow transplantation model, the differential migration of CRK/CRKL-deficient T cells resulted in efficient graft-versus-leukemia responses with minimal GVHD. Together, the results from our studies show that CRK family proteins selectively regulate T cell adhesion and migration at effector sites and suggest that these proteins have potential as therapeutic targets for preventing GVHD.
in vitro T cell stimulation/activation
in vitro T cell stimulation/activation
Huang, Y., et al (2015). "CRK proteins selectively regulate T cell migration into inflamed tissues" J Clin Invest 125(3): 1019-1032.
PubMed
Effector T cell migration into inflamed sites greatly exacerbates tissue destruction and disease severity in inflammatory diseases, including graft-versus-host disease (GVHD). T cell migration into such sites depends heavily on regulated adhesion and migration, but the signaling pathways that coordinate these functions downstream of chemokine receptors are largely unknown. Using conditional knockout mice, we found that T cells lacking the adaptor proteins CRK and CRK-like (CRKL) exhibit reduced integrin-dependent adhesion, chemotaxis, and diapedesis. Moreover, these two closely related proteins exhibited substantial functional redundancy, as ectopic expression of either protein rescued defects in T cells lacking both CRK and CRKL. We determined that CRK proteins coordinate with the RAP guanine nucleotide exchange factor C3G and the adhesion docking molecule CASL to activate the integrin regulatory GTPase RAP1. CRK proteins were required for effector T cell trafficking into sites of inflammation, but not for migration to lymphoid organs. In a murine bone marrow transplantation model, the differential migration of CRK/CRKL-deficient T cells resulted in efficient graft-versus-leukemia responses with minimal GVHD. Together, the results from our studies show that CRK family proteins selectively regulate T cell adhesion and migration at effector sites and suggest that these proteins have potential as therapeutic targets for preventing GVHD.
in vitro T cell stimulation/activation
in vivo CD8+ T cell depletion
in vitro T cell stimulation/activation
in vivo IL-9 neutralization
in vivo IL-10 neutralization
Vegran, F., et al (2014). "The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells" Nat Immunol 15(8): 758-766.
PubMed
The TH9 subset of helper T cells was initially shown to contribute to the induction of autoimmune and allergic diseases, but subsequent evidence has suggested that these cells also exert antitumor activities. However, the molecular events that account for their effector properties are elusive. Here we found that the transcription factor IRF1 enhanced the effector function of TH9 cells and dictated their anticancer properties. Under TH9-skewing conditions, interleukin 1beta (IL-1beta) induced phosphorylation of the transcription factor STAT1 and subsequent expression of IRF1, which bound to the promoters of Il9 and Il21 and enhanced secretion of the cytokines IL-9 and IL-21 from TH9 cells. Furthermore, IL-1beta-induced TH9 cells exerted potent anticancer functions in an IRF1- and IL-21-dependent manner. Our findings thus identify IRF1 as a target for controlling the function of TH9 cells.
in vitro T cell stimulation/activation
Bertin, S., et al (2015). "Dual-specificity phosphatase 6 regulates CD4+ T-cell functions and restrains spontaneous colitis in IL-10-deficient mice" Mucosal Immunol 8(3): 505-515.
PubMed
Mitogen-activated protein kinase (MAPK) phosphatases are dual-specificity phosphatases (DUSPs) that dephosphorylate phosphothreonine and phosphotyrosine residues within MAPKs. DUSP6 preferentially dephosphorylates extracellular signal-regulated kinases 1 and 2 (ERK1/2) rendering them inactive. Here, we study the role of DUSP6 in CD4(+) T-cell function, differentiation, and inflammatory profile in the colon. Upon T-cell receptor (TCR) stimulation, DUSP6 knockout (Dusp6(-/-)) CD4(+) T cells showed increased ERK1/2 activation, proliferation, T helper 1 differentiation, and interferon-gamma production, as well as a marked decrease in survival, interleukin- 17A (IL-17A) secretion, and regulatory T-cell function. To analyze the role of DUSP6 in vivo, we employed the Il10(-/-) model of colitis and generated Il10(-/-)/Dusp6(-/-) double-knockout mice. Il10(-/-)/Dusp6(-/-) mice suffered from accelerated and exacerbated spontaneous colitis, which was prevented by ERK1/2 inhibition. ERK1/2 inhibition also augmented regulatory T-cell differentiation in vitro and in vivo in both C57Bl/6 and Dusp6(-/-) mice. In summary, DUSP6 regulates CD4(+) T-cell activation and differentiation by inhibiting the TCR-dependent ERK1/2 activation. DUSP6 might therefore be a potential intervention target for limiting aberrant T-cell responses in T-cell-mediated diseases, such as inflammatory bowel disease.
in vitro T cell stimulation/activation
Nowak, E. C., et al (2009). "IL-9 as a mediator of Th17-driven inflammatory disease" J Exp Med 206(8): 1653-1660.
PubMed
We report that like other T cells cultured in the presence of transforming growth factor (TGF) beta, Th17 cells also produce interleukin (IL) 9. Th17 cells generated in vitro with IL-6 and TGF-beta as well as purified ex vivo Th17 cells both produced IL-9. To determine if IL-9 has functional consequences in Th17-mediated inflammatory disease, we evaluated the role of IL-9 in the development and progression of experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. The data show that IL-9 neutralization and IL-9 receptor deficiency attenuates disease, and this correlates with decreases in Th17 cells and IL-6-producing macrophages in the central nervous system, as well as mast cell numbers in the regional lymph nodes. Collectively, these data implicate IL-9 as a Th17-derived cytokine that can contribute to inflammatory disease.
in vitro T cell stimulation/activation
Chen, E. J., et al (2013). "Ezrin and moesin are required for efficient T cell adhesion and homing to lymphoid organs" PLoS One 8(2): e52368.
PubMed
T cell trafficking between the blood and lymphoid organs is a complex, multistep process that requires several highly dynamic and coordinated changes in cyto-architecture. Members of the ezrin, radixin and moesin (ERM) family of actin-binding proteins have been implicated in several aspects of this process, but studies have yielded conflicting results. Using mice with a conditional deletion of ezrin in CD4+ cells and moesin-specific siRNA, we generated T cells lacking ERM proteins, and investigated the effect on specific events required for T cell trafficking. ERM-deficient T cells migrated normally in multiple in vitro and in vivo assays, and could undergo efficient diapedesis in vitro. However, these cells were impaired in their ability to adhere to the beta1 integrin ligand fibronectin, and to polarize appropriately in response to fibronectin and VCAM-1 binding. This defect was specific for beta1 integrins, as adhesion and polarization in response to ICAM-1 were normal. In vivo, ERM-deficient T cells showed defects in homing to lymphoid organs. Taken together, these results show that ERM proteins are largely dispensable for T cell chemotaxis, but are important for beta1 integrin function and homing to lymphoid organs.
in vitro T cell stimulation/activation
Pallandre, J. R., et al (2015). "Novel aminotetrazole derivatives as selective STAT3 non-peptide inhibitors" Eur J Med Chem 103: 163-174.
PubMed
The development of inhibitors blocking STAT3 transcriptional activity is a promising therapeutic approach against cancer and inflammatory diseases. In this context, the selectivity of inhibitors against the STAT1 transcription factor is crucial as STAT3 and STAT1 play opposite roles in the apoptosis of tumor cells and polarization of the immune response. A structure-based virtual screening followed by a luciferase-containing promoter assay on STAT3 and STAT1 signaling were used to identify a selective STAT3 inhibitor. An important role of the aminotetrazole group in modulating STAT3 and STAT1 inhibitory activities has been established. Optimization of the hit compound leads to 23. This compound inhibits growth and survival of cells with STAT3 signaling pathway while displaying a minimal effect on STAT1 signaling. Moreover, it prevents lymphocyte T polarization into Th17 and Treg without affecting their differentiation into Th1 lymphocyte.
in vitro T cell stimulation/activation
Klimatcheva, E., et al (2015). "CXCL13 antibody for the treatment of autoimmune disorders" BMC Immunol 16: 6.
PubMed
BACKGROUND: Homeostatic B Cell-Attracting chemokine 1 (BCA-1) otherwise known as CXCL13 is constitutively expressed in secondary lymphoid organs by follicular dendritic cells (FDC) and macrophages. It is the only known ligand for the CXCR5 receptor, which is expressed on mature B cells, follicular helper T cells (Tfh), Th17 cells and regulatory T (Treg) cells. Aberrant expression of CXCL13 within ectopic germinal centers has been linked to the development of autoimmune disorders (e.g. Rheumatoid Arthritis, Multiple Sclerosis, Systemic Lupus Erythematosis). We, therefore, hypothesized that antibody-mediated disruption of the CXCL13 signaling pathway would interfere with the formation of ectopic lymphoid follicles in the target organs and inhibit autoimmune disease progression. This work describes pre-clinical development of human anti-CXCL13 antibody MAb 5261 and includes therapeutic efficacy data of its mouse counterpart in murine models of autoimmunity. RESULTS: We developed a human IgG1 monoclonal antibody, MAb 5261 that specifically binds to human, rodent and primate CXCL13 with an affinity of approximately 5 nM and is capable of neutralizing the activity of CXCL13 from these various species in in vitro functional assays. For in vivo studies we have engineered a chimeric antibody to contain the same human heavy and light chain variable genes along with mouse constant regions. Treatment with this antibody led to a reduction in the number of germinal centers in mice immunized with 4-Hydroxy-3-nitrophenylacetyl hapten conjugated to Keyhole Limpet Hemocyanin (NP-KLH) and, in adoptive transfer studies, interfered with the trafficking of B cells to the B cell areas of mouse spleen. Furthermore, this mouse anti-CXCL13 antibody demonstrated efficacy in a mouse model of Rheumatoid arthritis (Collagen-Induced Arthritis (CIA)) and Th17-mediated murine model of Multiple Sclerosis (passively-induced Experimental Autoimmune Encephalomyelitis (EAE)). CONCLUSIONS: We developed a novel therapeutic antibody targeting CXCL13-mediated signaling pathway for the treatment of autoimmune disorders.
in vitro T cell stimulation/activation
Bertin, S., et al (2015). "Dual-specificity phosphatase 6 regulates CD4+ T-cell functions and restrains spontaneous colitis in IL-10-deficient mice" Mucosal Immunol 8(3): 505-515.
PubMed
Mitogen-activated protein kinase (MAPK) phosphatases are dual-specificity phosphatases (DUSPs) that dephosphorylate phosphothreonine and phosphotyrosine residues within MAPKs. DUSP6 preferentially dephosphorylates extracellular signal-regulated kinases 1 and 2 (ERK1/2) rendering them inactive. Here, we study the role of DUSP6 in CD4(+) T-cell function, differentiation, and inflammatory profile in the colon. Upon T-cell receptor (TCR) stimulation, DUSP6 knockout (Dusp6(-/-)) CD4(+) T cells showed increased ERK1/2 activation, proliferation, T helper 1 differentiation, and interferon-gamma production, as well as a marked decrease in survival, interleukin- 17A (IL-17A) secretion, and regulatory T-cell function. To analyze the role of DUSP6 in vivo, we employed the Il10(-/-) model of colitis and generated Il10(-/-)/Dusp6(-/-) double-knockout mice. Il10(-/-)/Dusp6(-/-) mice suffered from accelerated and exacerbated spontaneous colitis, which was prevented by ERK1/2 inhibition. ERK1/2 inhibition also augmented regulatory T-cell differentiation in vitro and in vivo in both C57Bl/6 and Dusp6(-/-) mice. In summary, DUSP6 regulates CD4(+) T-cell activation and differentiation by inhibiting the TCR-dependent ERK1/2 activation. DUSP6 might therefore be a potential intervention target for limiting aberrant T-cell responses in T-cell-mediated diseases, such as inflammatory bowel disease.
in vitro T cell stimulation/activation
Nowak, E. C., et al (2009). "IL-9 as a mediator of Th17-driven inflammatory disease" J Exp Med 206(8): 1653-1660.
PubMed
We report that like other T cells cultured in the presence of transforming growth factor (TGF) beta, Th17 cells also produce interleukin (IL) 9. Th17 cells generated in vitro with IL-6 and TGF-beta as well as purified ex vivo Th17 cells both produced IL-9. To determine if IL-9 has functional consequences in Th17-mediated inflammatory disease, we evaluated the role of IL-9 in the development and progression of experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. The data show that IL-9 neutralization and IL-9 receptor deficiency attenuates disease, and this correlates with decreases in Th17 cells and IL-6-producing macrophages in the central nervous system, as well as mast cell numbers in the regional lymph nodes. Collectively, these data implicate IL-9 as a Th17-derived cytokine that can contribute to inflammatory disease.
in vitro T cell stimulation/activation
Chen, E. J., et al (2013). "Ezrin and moesin are required for efficient T cell adhesion and homing to lymphoid organs" PLoS One 8(2): e52368.
PubMed
T cell trafficking between the blood and lymphoid organs is a complex, multistep process that requires several highly dynamic and coordinated changes in cyto-architecture. Members of the ezrin, radixin and moesin (ERM) family of actin-binding proteins have been implicated in several aspects of this process, but studies have yielded conflicting results. Using mice with a conditional deletion of ezrin in CD4+ cells and moesin-specific siRNA, we generated T cells lacking ERM proteins, and investigated the effect on specific events required for T cell trafficking. ERM-deficient T cells migrated normally in multiple in vitro and in vivo assays, and could undergo efficient diapedesis in vitro. However, these cells were impaired in their ability to adhere to the beta1 integrin ligand fibronectin, and to polarize appropriately in response to fibronectin and VCAM-1 binding. This defect was specific for beta1 integrins, as adhesion and polarization in response to ICAM-1 were normal. In vivo, ERM-deficient T cells showed defects in homing to lymphoid organs. Taken together, these results show that ERM proteins are largely dispensable for T cell chemotaxis, but are important for beta1 integrin function and homing to lymphoid organs.
in vitro T cell stimulation/activation
Pallandre, J. R., et al (2015). "Novel aminotetrazole derivatives as selective STAT3 non-peptide inhibitors" Eur J Med Chem 103: 163-174.
PubMed
The development of inhibitors blocking STAT3 transcriptional activity is a promising therapeutic approach against cancer and inflammatory diseases. In this context, the selectivity of inhibitors against the STAT1 transcription factor is crucial as STAT3 and STAT1 play opposite roles in the apoptosis of tumor cells and polarization of the immune response. A structure-based virtual screening followed by a luciferase-containing promoter assay on STAT3 and STAT1 signaling were used to identify a selective STAT3 inhibitor. An important role of the aminotetrazole group in modulating STAT3 and STAT1 inhibitory activities has been established. Optimization of the hit compound leads to 23. This compound inhibits growth and survival of cells with STAT3 signaling pathway while displaying a minimal effect on STAT1 signaling. Moreover, it prevents lymphocyte T polarization into Th17 and Treg without affecting their differentiation into Th1 lymphocyte.
in vitro T cell stimulation/activation
Klimatcheva, E., et al (2015). "CXCL13 antibody for the treatment of autoimmune disorders" BMC Immunol 16: 6.
PubMed
BACKGROUND: Homeostatic B Cell-Attracting chemokine 1 (BCA-1) otherwise known as CXCL13 is constitutively expressed in secondary lymphoid organs by follicular dendritic cells (FDC) and macrophages. It is the only known ligand for the CXCR5 receptor, which is expressed on mature B cells, follicular helper T cells (Tfh), Th17 cells and regulatory T (Treg) cells. Aberrant expression of CXCL13 within ectopic germinal centers has been linked to the development of autoimmune disorders (e.g. Rheumatoid Arthritis, Multiple Sclerosis, Systemic Lupus Erythematosis). We, therefore, hypothesized that antibody-mediated disruption of the CXCL13 signaling pathway would interfere with the formation of ectopic lymphoid follicles in the target organs and inhibit autoimmune disease progression. This work describes pre-clinical development of human anti-CXCL13 antibody MAb 5261 and includes therapeutic efficacy data of its mouse counterpart in murine models of autoimmunity. RESULTS: We developed a human IgG1 monoclonal antibody, MAb 5261 that specifically binds to human, rodent and primate CXCL13 with an affinity of approximately 5 nM and is capable of neutralizing the activity of CXCL13 from these various species in in vitro functional assays. For in vivo studies we have engineered a chimeric antibody to contain the same human heavy and light chain variable genes along with mouse constant regions. Treatment with this antibody led to a reduction in the number of germinal centers in mice immunized with 4-Hydroxy-3-nitrophenylacetyl hapten conjugated to Keyhole Limpet Hemocyanin (NP-KLH) and, in adoptive transfer studies, interfered with the trafficking of B cells to the B cell areas of mouse spleen. Furthermore, this mouse anti-CXCL13 antibody demonstrated efficacy in a mouse model of Rheumatoid arthritis (Collagen-Induced Arthritis (CIA)) and Th17-mediated murine model of Multiple Sclerosis (passively-induced Experimental Autoimmune Encephalomyelitis (EAE)). CONCLUSIONS: We developed a novel therapeutic antibody targeting CXCL13-mediated signaling pathway for the treatment of autoimmune disorders.
in vitro T cell stimulation/activation
Huang, Y., et al (2015). "CRK proteins selectively regulate T cell migration into inflamed tissues" J Clin Invest 125(3): 1019-1032.
PubMed
Effector T cell migration into inflamed sites greatly exacerbates tissue destruction and disease severity in inflammatory diseases, including graft-versus-host disease (GVHD). T cell migration into such sites depends heavily on regulated adhesion and migration, but the signaling pathways that coordinate these functions downstream of chemokine receptors are largely unknown. Using conditional knockout mice, we found that T cells lacking the adaptor proteins CRK and CRK-like (CRKL) exhibit reduced integrin-dependent adhesion, chemotaxis, and diapedesis. Moreover, these two closely related proteins exhibited substantial functional redundancy, as ectopic expression of either protein rescued defects in T cells lacking both CRK and CRKL. We determined that CRK proteins coordinate with the RAP guanine nucleotide exchange factor C3G and the adhesion docking molecule CASL to activate the integrin regulatory GTPase RAP1. CRK proteins were required for effector T cell trafficking into sites of inflammation, but not for migration to lymphoid organs. In a murine bone marrow transplantation model, the differential migration of CRK/CRKL-deficient T cells resulted in efficient graft-versus-leukemia responses with minimal GVHD. Together, the results from our studies show that CRK family proteins selectively regulate T cell adhesion and migration at effector sites and suggest that these proteins have potential as therapeutic targets for preventing GVHD.
in vitro T cell stimulation/activation
Bertin, S., et al (2015). "Dual-specificity phosphatase 6 regulates CD4+ T-cell functions and restrains spontaneous colitis in IL-10-deficient mice" Mucosal Immunol 8(3): 505-515.
PubMed
Mitogen-activated protein kinase (MAPK) phosphatases are dual-specificity phosphatases (DUSPs) that dephosphorylate phosphothreonine and phosphotyrosine residues within MAPKs. DUSP6 preferentially dephosphorylates extracellular signal-regulated kinases 1 and 2 (ERK1/2) rendering them inactive. Here, we study the role of DUSP6 in CD4(+) T-cell function, differentiation, and inflammatory profile in the colon. Upon T-cell receptor (TCR) stimulation, DUSP6 knockout (Dusp6(-/-)) CD4(+) T cells showed increased ERK1/2 activation, proliferation, T helper 1 differentiation, and interferon-gamma production, as well as a marked decrease in survival, interleukin- 17A (IL-17A) secretion, and regulatory T-cell function. To analyze the role of DUSP6 in vivo, we employed the Il10(-/-) model of colitis and generated Il10(-/-)/Dusp6(-/-) double-knockout mice. Il10(-/-)/Dusp6(-/-) mice suffered from accelerated and exacerbated spontaneous colitis, which was prevented by ERK1/2 inhibition. ERK1/2 inhibition also augmented regulatory T-cell differentiation in vitro and in vivo in both C57Bl/6 and Dusp6(-/-) mice. In summary, DUSP6 regulates CD4(+) T-cell activation and differentiation by inhibiting the TCR-dependent ERK1/2 activation. DUSP6 might therefore be a potential intervention target for limiting aberrant T-cell responses in T-cell-mediated diseases, such as inflammatory bowel disease.
in vitro T cell stimulation/activation
Klimatcheva, E., et al (2015). "CXCL13 antibody for the treatment of autoimmune disorders" BMC Immunol 16: 6.
PubMed
BACKGROUND: Homeostatic B Cell-Attracting chemokine 1 (BCA-1) otherwise known as CXCL13 is constitutively expressed in secondary lymphoid organs by follicular dendritic cells (FDC) and macrophages. It is the only known ligand for the CXCR5 receptor, which is expressed on mature B cells, follicular helper T cells (Tfh), Th17 cells and regulatory T (Treg) cells. Aberrant expression of CXCL13 within ectopic germinal centers has been linked to the development of autoimmune disorders (e.g. Rheumatoid Arthritis, Multiple Sclerosis, Systemic Lupus Erythematosis). We, therefore, hypothesized that antibody-mediated disruption of the CXCL13 signaling pathway would interfere with the formation of ectopic lymphoid follicles in the target organs and inhibit autoimmune disease progression. This work describes pre-clinical development of human anti-CXCL13 antibody MAb 5261 and includes therapeutic efficacy data of its mouse counterpart in murine models of autoimmunity. RESULTS: We developed a human IgG1 monoclonal antibody, MAb 5261 that specifically binds to human, rodent and primate CXCL13 with an affinity of approximately 5 nM and is capable of neutralizing the activity of CXCL13 from these various species in in vitro functional assays. For in vivo studies we have engineered a chimeric antibody to contain the same human heavy and light chain variable genes along with mouse constant regions. Treatment with this antibody led to a reduction in the number of germinal centers in mice immunized with 4-Hydroxy-3-nitrophenylacetyl hapten conjugated to Keyhole Limpet Hemocyanin (NP-KLH) and, in adoptive transfer studies, interfered with the trafficking of B cells to the B cell areas of mouse spleen. Furthermore, this mouse anti-CXCL13 antibody demonstrated efficacy in a mouse model of Rheumatoid arthritis (Collagen-Induced Arthritis (CIA)) and Th17-mediated murine model of Multiple Sclerosis (passively-induced Experimental Autoimmune Encephalomyelitis (EAE)). CONCLUSIONS: We developed a novel therapeutic antibody targeting CXCL13-mediated signaling pathway for the treatment of autoimmune disorders.
in vitro T cell stimulation/activation
Pallandre, J. R., et al (2015). "Novel aminotetrazole derivatives as selective STAT3 non-peptide inhibitors" Eur J Med Chem 103: 163-174.
PubMed
The development of inhibitors blocking STAT3 transcriptional activity is a promising therapeutic approach against cancer and inflammatory diseases. In this context, the selectivity of inhibitors against the STAT1 transcription factor is crucial as STAT3 and STAT1 play opposite roles in the apoptosis of tumor cells and polarization of the immune response. A structure-based virtual screening followed by a luciferase-containing promoter assay on STAT3 and STAT1 signaling were used to identify a selective STAT3 inhibitor. An important role of the aminotetrazole group in modulating STAT3 and STAT1 inhibitory activities has been established. Optimization of the hit compound leads to 23. This compound inhibits growth and survival of cells with STAT3 signaling pathway while displaying a minimal effect on STAT1 signaling. Moreover, it prevents lymphocyte T polarization into Th17 and Treg without affecting their differentiation into Th1 lymphocyte.
in vitro T cell stimulation/activation
Bertin, S., et al (2014). "The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4(+) T cells" Nat Immunol 15(11): 1055-1063.
PubMed
TRPV1 is a Ca(2+)-permeable channel studied mostly as a pain receptor in sensory neurons. However, its role in other cell types is poorly understood. Here we found that TRPV1 was functionally expressed in CD4(+) T cells, where it acted as a non-store-operated Ca(2+) channel and contributed to T cell antigen receptor (TCR)-induced Ca(2+) influx, TCR signaling and T cell activation. In models of T cell-mediated colitis, TRPV1 promoted colitogenic T cell responses and intestinal inflammation. Furthermore, genetic and pharmacological inhibition of TRPV1 in human CD4(+) T cells recapitulated the phenotype of mouse Trpv1(-/-) CD4(+) T cells. Our findings suggest that inhibition of TRPV1 could represent a new therapeutic strategy for restraining proinflammatory T cell responses.
in vitro T cell stimulation/activation
Vegran, F., et al (2014). "The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells" Nat Immunol 15(8): 758-766.
PubMed
The TH9 subset of helper T cells was initially shown to contribute to the induction of autoimmune and allergic diseases, but subsequent evidence has suggested that these cells also exert antitumor activities. However, the molecular events that account for their effector properties are elusive. Here we found that the transcription factor IRF1 enhanced the effector function of TH9 cells and dictated their anticancer properties. Under TH9-skewing conditions, interleukin 1beta (IL-1beta) induced phosphorylation of the transcription factor STAT1 and subsequent expression of IRF1, which bound to the promoters of Il9 and Il21 and enhanced secretion of the cytokines IL-9 and IL-21 from TH9 cells. Furthermore, IL-1beta-induced TH9 cells exerted potent anticancer functions in an IRF1- and IL-21-dependent manner. Our findings thus identify IRF1 as a target for controlling the function of TH9 cells.
in vitro T cell stimulation/activation
Berger, H., et al (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590.
PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vitro T cell stimulation/activation
Chen, E. J., et al (2013). "Ezrin and moesin are required for efficient T cell adhesion and homing to lymphoid organs" PLoS One 8(2): e52368.
PubMed
T cell trafficking between the blood and lymphoid organs is a complex, multistep process that requires several highly dynamic and coordinated changes in cyto-architecture. Members of the ezrin, radixin and moesin (ERM) family of actin-binding proteins have been implicated in several aspects of this process, but studies have yielded conflicting results. Using mice with a conditional deletion of ezrin in CD4+ cells and moesin-specific siRNA, we generated T cells lacking ERM proteins, and investigated the effect on specific events required for T cell trafficking. ERM-deficient T cells migrated normally in multiple in vitro and in vivo assays, and could undergo efficient diapedesis in vitro. However, these cells were impaired in their ability to adhere to the beta1 integrin ligand fibronectin, and to polarize appropriately in response to fibronectin and VCAM-1 binding. This defect was specific for beta1 integrins, as adhesion and polarization in response to ICAM-1 were normal. In vivo, ERM-deficient T cells showed defects in homing to lymphoid organs. Taken together, these results show that ERM proteins are largely dispensable for T cell chemotaxis, but are important for beta1 integrin function and homing to lymphoid organs.
in vitro T cell stimulation/activation
Nowak, E. C., et al (2009). "IL-9 as a mediator of Th17-driven inflammatory disease" J Exp Med 206(8): 1653-1660.
PubMed
We report that like other T cells cultured in the presence of transforming growth factor (TGF) beta, Th17 cells also produce interleukin (IL) 9. Th17 cells generated in vitro with IL-6 and TGF-beta as well as purified ex vivo Th17 cells both produced IL-9. To determine if IL-9 has functional consequences in Th17-mediated inflammatory disease, we evaluated the role of IL-9 in the development and progression of experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. The data show that IL-9 neutralization and IL-9 receptor deficiency attenuates disease, and this correlates with decreases in Th17 cells and IL-6-producing macrophages in the central nervous system, as well as mast cell numbers in the regional lymph nodes. Collectively, these data implicate IL-9 as a Th17-derived cytokine that can contribute to inflammatory disease.